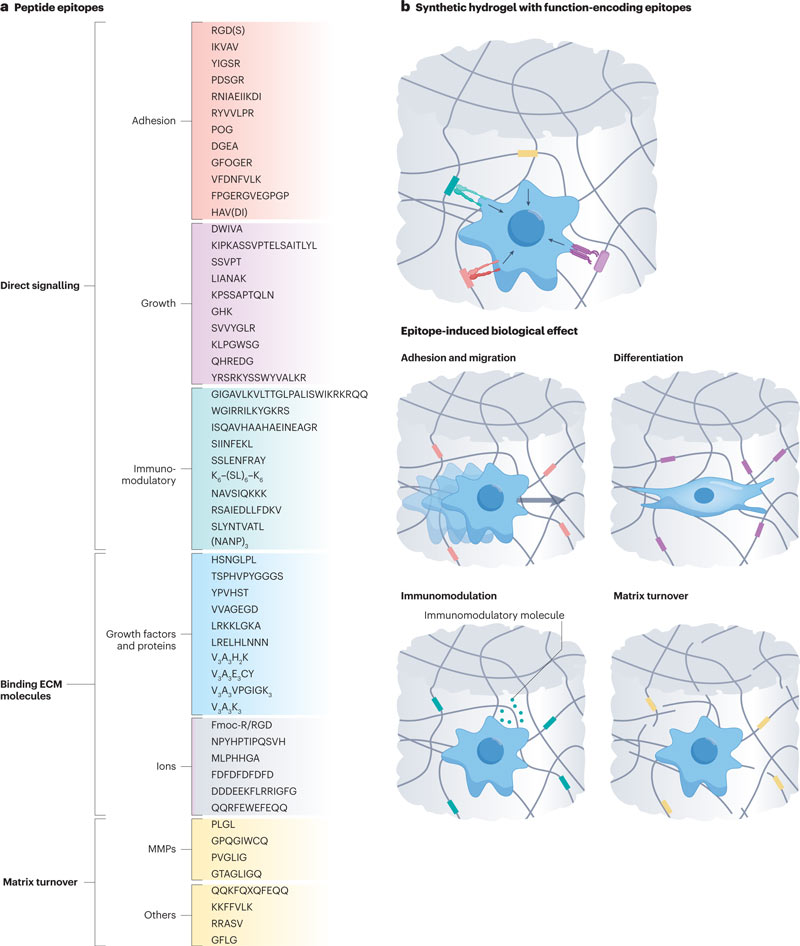
Protein epitopes can be used as the basis for building cell-directed biomaterials. Proteins are made up of chains of amino acids called peptides and are called epitopes if they contain bioactive sequences capable of interacting with specific cell receptors or antibodies.
outline
- Communication between cells and their extracellular matrix (ECM) is encoded in the peptide epitopes of structural and signaling ECM proteins, which act as molecular “coordinators” for a variety of cellular functions.
- Peptide epitopes can be integrated into biomaterials to achieve selective and specific communication with cells and tissues.
- Designing biomaterials with specific peptide epitopes can control cell adhesion, differentiation, immune regulation, and extracellular matrix turnover.
- The peptide epitope molecular toolbox can be used for the design of functional synthetic ECMs in tissue engineering and bioengineering applications.
introduction
These short amino acid sequences are the main “functional coding language” of proteins and can be used as building blocks for biological materials.
In particular, self-assembling peptides are molecular building blocks that have a high tendency to self-assemble into ECM-like nanofibers and hydrogels in aqueous environments.
This material platform enables the presentation of peptide epitopes on nanofiber surfaces to actively interact with cells, initiate specific cell signaling pathways, act as therapeutic agents, or target cells and tissues in vivo.
The multi-purpose molecular design of the self-assembling peptides also allows 3D nanofiber hydrogels to have a range of mechanical, chemical and biological properties for cell culture, cell delivery and biofabrication.
This paper mainly discusses the design of biomaterials based on peptides as signal building blocks.
A peptide epitope library is provided, which can be used as a molecular toolbox for bioengineering of synthetic hydrogels, with biological functions such as structural support, signal transduction and dynamics.
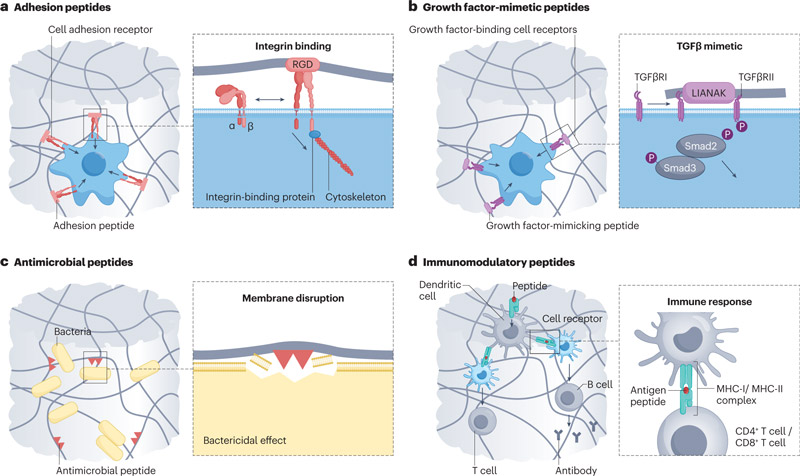
Polypeptides: direct signaling
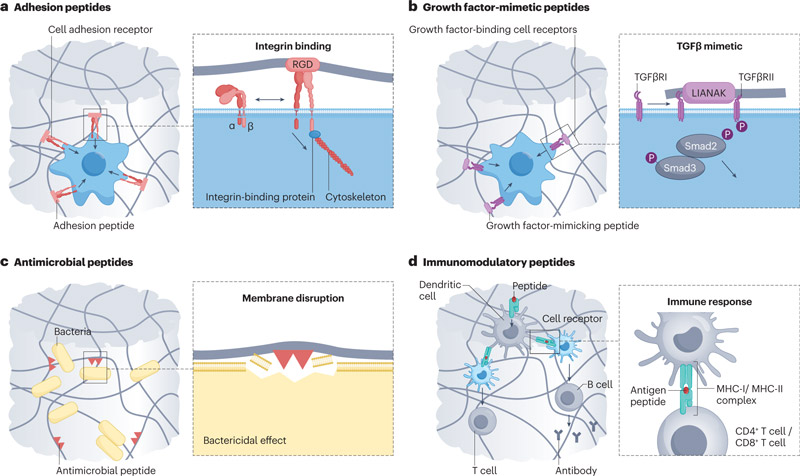
1. Peptide sequences that promote cell adhesion and growth
a. Cell adhesion epitopes derived from fibronectin
RGD peptide exists in the fifinexin domain FNIII9-10, and is one of the most studied cell adhesion peptide sequences. The incorporation of RGD peptide makes the synthesized hydrogels have cell adhesion properties.
b. Cell adhesion epitopes are derived from laminin
Lamin-derived peptides, including IKVAV on the lamin-α-chain, YIGSR, PDSGR, RYVVLPR on the lamin-β-chain, and RNIAEIIKDI on the lamin-γ-chain, can be used to induce cell aggregation and cluster formation in angiogenesis and neurogenesis.
c. tendine-derived cell adhesion epitopes
Tendinin is a class of glycoproteins involved in neurogenesis, osteogenesis and angiogenesis, and its derived peptides can be used as functional coding tools to promote cell adhesion.
For example, tendine-derived octapeptide VFDNFVLK.
d. Cell adhesion epitopes derived from collagen
Collagen provides structural support for hard and soft tissues and binds to integrins to support cell attachment.
The collagen superfamily consists of 46 different polypeptide chains, which are supramolecular assembled into 28 types of collagen according to their hierarchical organization and function.
For example, the colloidal peptide KOD ((PKG)4-(POG)4-(DOG)4) establishes a strong lysine-aspartate bridge hydrogen bond and can be assembled into a stable triple helix structure in layers.
The colloid-derived peptides DGEA and GFOGER have been studied in bone tissue engineering.
Collagen type I epitope FPGER, as part of the FPGERGVEGPGP peptide, regulates the migration and proliferation of keratinocytes and dermal fibroblasts in vitro.
e. Cell adhesion epitopes derived from cadherin
Cadherin is a transmembrane cell receptor responsible for intercellular signaling, cell migration and maintenance of tissue structure.
For example, N-cadherin is essential for mesenchymal stem cell aggregation, a key step in cartilage morphogenesis.
Cadherin contains the evolutionarily conserved tripeptide HAV, which is an important intercellular adhesion mediator.
HAVDI, a pentapeptide located in N-cadherin extracellular domain 1, triggers chondrogenesis in human stem cells in vitro by inhibiting typical Wnt/β-catenin signaling.
f. Other cell adhesion epitopes targeting integrins
Given the critical role of integrin binding in mediating angiogenesis, osteogenesis, and wound healing, integrin targeting is a key consideration in the design of many biomaterials.
Examples include GFPGER, RRETAWA, AGQWHRVSVRWG.
In most published studies, RGD remains the best-studied integrin-binding peptide (89%), followed by IKVAV (6%), YIGSR (4%), and other sequences (1%).

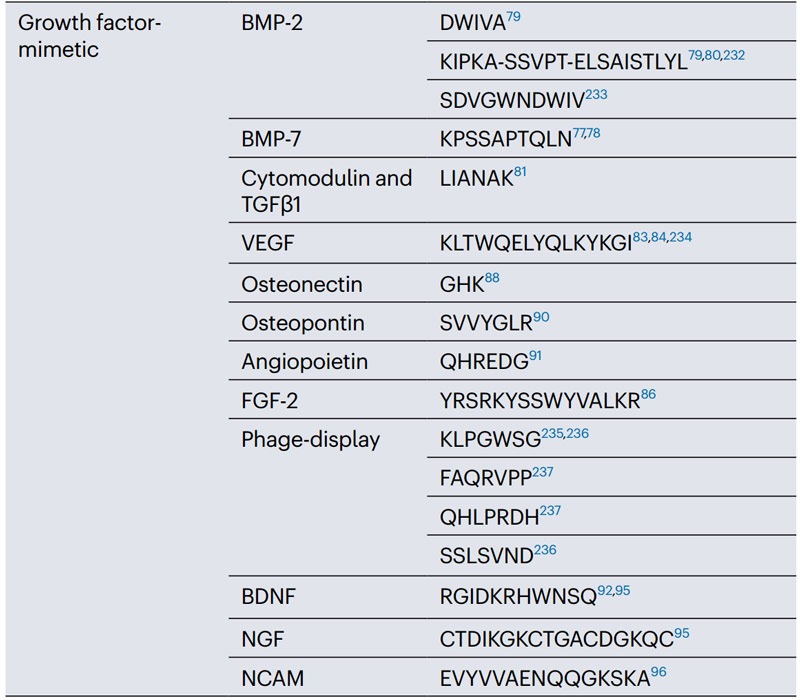
2. Polypeptide sequence of simulated growth factors
Growth factors are involved in many key cellular processes such as cell proliferation, migration, and differentiation, and they coordinate embryonic development, tissue homeostasis, and repair by influencing and directing stem cell phenotypes.
For example, bone morphogenetic proteins (BMPs) play a key role in osteogenesis, vascular endothelial growth factor (VEGF) promotes angiogenesis, and transforming growth factor (TGFβs) are important regulators of cartilage formation.
Simulated growth factor peptides can be added to 3D synthetic hydrogels to avoid rapid denaturation and clearance of full-length growth factors while ensuring reactivity, specificity, and efficiency.
a. Growth factor mimicking peptides for osteogenesis, cartilage and disc formation
Bmp-derived peptides can be used for osteogenic applications.
BMP is a multifunctional growth factor in the TGFβ superfamily that plays a crucial role in heart, brain, cartilage and bone development.
For example, the peptide epitope KPSSAPTQLN derived from BMP-7, and the peptide DWIVA derived from BMP-2.
LIANAK is a peptide that mimics TGFβ1 to increase cartilage gene expression and ECM deposition.
b. Angiogenic growth factor mimicking peptide
KLTWQELYQLKYKGI (KLT), a VEGF-mimicry peptide from VEGF helix region 17-35, binds to VEGF receptors, activates cell signaling and promotes capillary formation.
In chicken embryo chorio-allantoic membrane experiments, KLT-modified RADA16-I hydrogel promoted endothelial cell growth, induced the formation of two – and three-dimensional capillary-like tubules, and the formation of new blood vessels.
Basic fibroblast growth factor (bFGF), also known as FGF-2, also has a variety of roles, including angiogenesis and wound healing. The peptide domain 106-120 in FGF-2 is a partial agonist for the FGF receptor.
FGF-2 simulated peptide YRSRKYSSWYVALKR can be used to prepare angiogenic peptide nanoribbon hydrogels, which can promote the proliferation and migration of human umbilical vein endothelial cells in vitro.
Osteoconnexin-derived GHK tripeptides also have angiogenic effects.
SVVYGLR, a heptapeptide derived from osteopontin, coupled with RADA16 hydrogel, can stimulate in vitro angiogenesis of rat lung endothelial cells at a level comparable to VEGF, which may be used in the treatment of myocardial infarction.
c. Growth factor mimicking peptides for neurogenesis
Synthetic hydrogels can be used to promote nerve regeneration.
Nerve growth factor (NGF) promotes neurite growth, regulates peripheral sensory and sympathetic cell function, and brain-derived growth factor (BDNF) provides neuroprotection and regeneration of injured neurons.
The peptides CTDIKGKCTGACDGKQC and RGIDKRHWNSQ derived from NGF and BDNF, respectively, promote neurite proliferation and neuroprotective effects, similar to full-length growth factors.
3. Peptide sequences that induce immunomodulatory effects
a. Peptide hydrogels with natural antibacterial effects
Due to the presence of positive charges, cationic amphotereptides have intrinsic antimicrobial properties that promote interactions and cross-pollination within negatively charged bacterial membranes, leading to bacterial cell death.
For example, the antimicrobial peptide (AMP) WGIRRILKYGKRS (WMR) has an additional tryptophan amino acid and more positively charged arginine residues in the n-terminal, which makes the sequence have strong antibacterial activity.
Melittin derived peptide GIGAVLKVLTTGLPALISWIKRKRQQ also can be used as antibacterial coating.
The biocompatible bacterial membrane breaking self-assembly peptide system (CASP-K6) containing multiple lysine residues has a strong bactericidal effect on P. aeruginosa.
The presence of lysine in β-rich sheet hexapeptides (NAVSIQKKK) linked by lysine tripeptides leads to antimicrobial activity against Escherichia coli and Staphylococcus aureus.
b. Peptide hydrogels with inherent antibacterial effects
Injectable hydrogels can be made antibacterial by incorporating peptides with antimicrobial properties. Such as PEP6R, MAX1, and MARG1.
Low molecular weight peptide hydrogels based on Fmoc-FFE and Nap-FFE peptides also have bactericidal effects against Staphylococcus aureus and Staphylococcus epidermidis.
NapFFKK and NapFFFKK showed antibiofilm activity against Gram-positive and Gram-negative strains.
c. Peptide hydrogels that enhance the immune response of T and B cells
Peptides can also be used as chemically defined immune adjuvants;
For example, a peptide system (O-Q11) based on the Q11 self-assembly domain in tandem with the ovalbumen region (i.e. OVA323-339) contains antigenic epitopes recognized by T and B cells that self-assemble into nanofibers under physiological conditions.
Intranasal or subcutaneous administration of the nanofibers elicited a strong immune response in mice without adjuvants, elicited high levels of immunoglobulin, and promoted T cell activation that, if bound to the H1N1 influenza epitope, elicited an antigenic response
d. Peptide hydrogels for immunity to viruses
Peptide vaccines are based on peptide epitopes that trigger an immune response against a specific virus.
For example, the self-assembled polypeptide amphiphilic system enhances immunity to enterovirus 71 (EV71), a picorbonaviridae virus that causes hand, foot and mouth disease.
Peptide amphiphilic nanofibers display two antigenic epitopes of EV71, viral particle 1 (YPTFGEHKQEKDLEY) and 3 (HYRAHARDGVFDYYT), leading to humoral and cellular immune responses in mice.
The serum of these immunized mice neutralized EV71 and protected host cells.
Polypeptides: Bind ECM
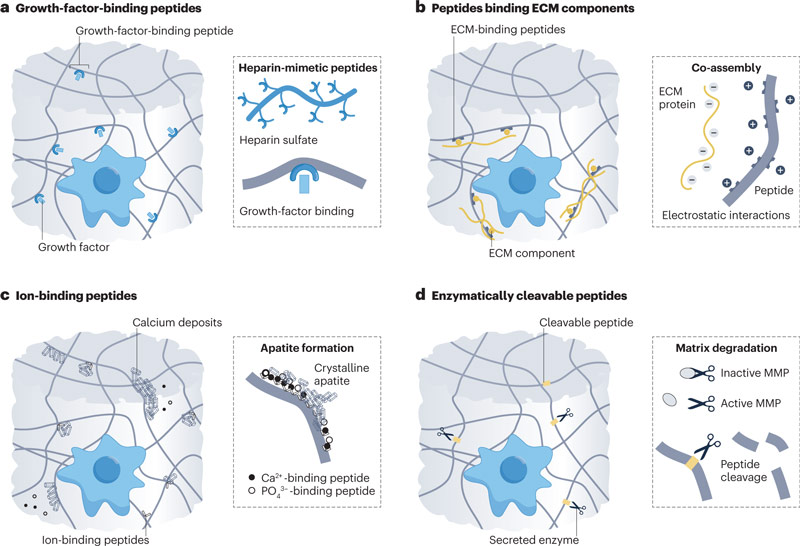
1. Binding growth factor peptide sequence
a. Peptides that directly bind to growth factors
Soluble growth factors commonly used in vitro and clinically to guide cell fate.
They usually supplied in superphysiological doses to ensure high concentrations and effectiveness for long periods, which can lead to off-target effects.
For example, YPVHST can bind BMP-2 and HSNGLPL can bind TGF-β1.
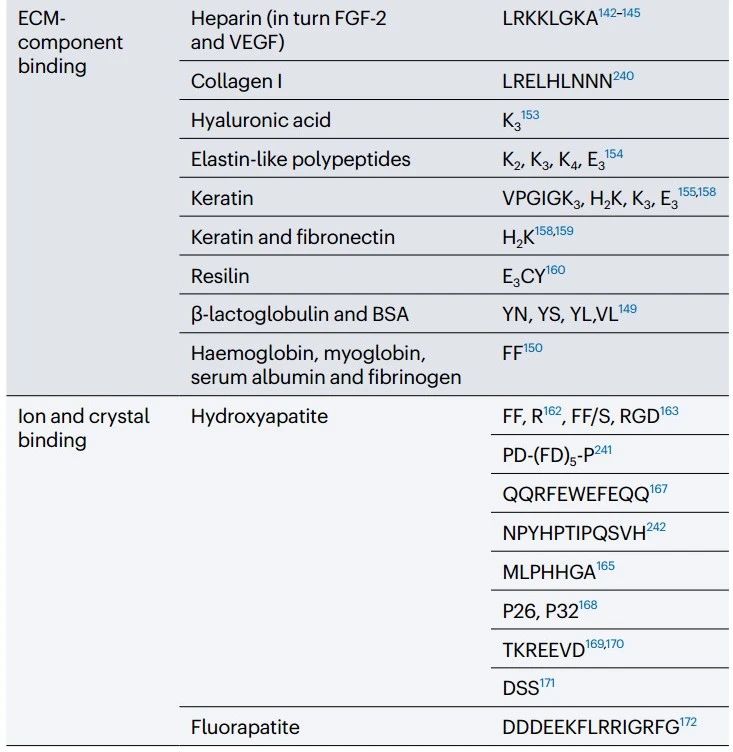
b. Polypeptides bound to growth factors by heparin simulation domains and heparin binding domains
Glycopeptidoides provide a versatile toolbox for binding multiple growth factors.
Glycans bind to more than 300 secreted or membrane binding proteins, such as growth factors, enzymes, and chemokines, to regulate a variety of biological processes.
For example, glycan heparin sulfate and heparin can hybridize with a variety of growth factors.
Interacts with heparin-sulfonic acid-binding growth factors such as BMP-2, BMP-4, FGF-1, FGF-2, VEGF, Shh, and growth factor inhibitor Noggin.
2. Peptide sequences that bind other ECM components
a. Peptides bind ECM molecules by selective binding
The ability to combine multiple molecular and biological components is key to simulating the complexity of natural tissue composition.
For example, polymethacrylate hydrogels modified with Hepsin-targeting peptide (IPLVVPL) selectively bind Hepsin-overexpressing tumor cells.
Nap-FFG peptides selectively bind and self-assemble into a coating on the surface of platelets, preventing human platelet aggregation in vitro.
Assembly of FMOC-dipeptides (i.e., Fmoc-YN, Fmoc-YS, Fmoc-YL, and Fmoc-VL) with beta-lactoglobulin and bovine serum albumin (BSA) can achieved by changing the polarity of the amino acids.
Ff-based hydrogels can also designed to self-assemble in the presence of hemoglobin, myoglobin, serum albumin, and fibrinogen by utilizing covalent Schiff base bonds.
Tyrosine and pyridine based gels can designed supramolecular systems that selectively bind nickel (Ni2+) and chloride ions (Cl−).
b. Peptides bind ECM molecules by non-selective binding
Peptides enable non-selective binding of ECM components based on complementary charge and hydrophobicity, allowing for the binding of multiple macromolecules and assembly of materials with high levels of structural hierarchy and function.
For example, the non-specific interaction of positively charged PA (V3A3K3) and negatively charged hyaluronic acid at the liquid-liquid interface leads to the formation of a diffusion barrier, followed by the co-assembly of the peptide and hyaluronic acid into structures of different sizes.
c. Polypeptide-binding ions regulate organic-inorganic interactions
The ECM of some tissues, such as bone and tooth enamel, is a nano-composite of organic and inorganic phases, which organized in layers to provide high strength and fracture toughness.
In these tissues, the local distribution and concentration of ions and minerals, such as calcium and phosphate deposits, are critical to ensuring structure and function.
For example, in bone, the organic phase is mostly type I collagen, while the inorganic phase is hydroxyapatite.
Polypeptides play an important role in the biomineralization process, directing organic-inorganic interactions.
In particular, hydroxyapatite aggregates and their crystal formation can regulated by the interaction of negatively and positively charged amino acids, such as glutamic acid and arginine, with Ca2+ and (PO4)3- ions.
Fmoc-FF, Fmoc-R and hydroxyapatite phases can combined into a multi-component peptide-based bone regeneration scaffold.
In this system, Fmoc-FF provides structural rigidity, while Fmoc-R acts as a template for biomineralization due to the presence of arginine amino acids.
These hydrogels containing hydroxyapatite are biocompatible and mechanically robust (hardness up to 30 kpa) and can therefore applied in cell culture as bionic scaffolds for bone regeneration research.
Fmoc-FF/S can also bind to Fmoc-RGD and hydroxyapatite nanocrystals, deposit hydroxyapatite from R and D amino acids on the RGD motif, and stimulate Raw 264.7 macrophages to differentiate into osteoclasts.
Peptide sequence can reasonably designed to guide biomineralization.
For example, dodecapeptide NPYHPTIPQSVH binds to the surface of hydroxyapatite crystals, and heptapeptide MLPHHGA binds to the β-hairpin formation sequence (MDG1), directing the formation of hydroxyapatite in the presence of cementioblast cells.
In tooth enamel, organic substrates play a crucial role in the development of layered inorganic structure and mechanical properties of tooth enamel.
Negatively charged macromolecules in enamel ECM, including glycoproteins and proteins such as amelogenin, enamelin, and amelotin, bind Ca2+ ions and trigger apatite crystal nucleation and growth.
To simulate this process, a low-viscosity solution of anionic P11-4 self-assembly peptide can designed that infiltrates and polymerizes into caries lesions to provide a biomimetic scaffold for hydroxyapatite nucleation.
This peptide increases enamel mineralization and deposition of newly crystallized hydroxyapatite in vitro and has achieved enamel mineralization in clinical safety trials.
Mimicking enamel glycoproteins, 26 – and 32-amino acid amylogenic activating peptides (P26 and P32) can engineered as hydrogels to promote the bottom-up formation of multiple layers of hydroxyapatite crystals in human molars.
Similarly, enamel remineralization can also achieved by shorter amelogenin mimics, such as LEAWPATDKTKREEVD and TKREEVD, as well as tripeptide motifs (DSS) from dentin phosphoproteins.
The peptide DDDEEKFLRRIGRFG, derived from steroid protein, a protein present in saliva, controls the formation of fluorapatite.
3. Peptide sequence creates dynamic matrix
a. Peptides cleaved by matrix metalloproteinases
In healthy tissues, deposition and degradation of ECM tightly regulated to ensure homeostasis and matrix turnover.
Matrix metalloproteinases (MMPs) zinc-dependent endopeptidases that often overexpressed in tissue remodeling and pathological states such as cancer.
The MMP-sensitive sequence GPX1G*LX2G (where * represents the cleavage site, X1 is A or L, X2 is G) can incorporated into the hydrogel to control its enzymatic degradation and generate a cleavable synthetic matrix.
For example, the MMP-2 sensitive sequence GTAGLIGQ can serve as a cleavable skeleton for PA hydrogels.
Similarly, the MMP-9 sensitive sequence GL in peptide FFAGLDD allows adriamycin to released in vitro from phenylacetyl PAs, capable of switching from micellar aggregates to fibers after degradation at MMP-sensitive sites.
A diphenylalanine-based peptide hydrogel, consisting of an MMP-sensitive tetrapeptide (PLGL) connected to a neuroprotective hexapeptide (NAVSIQ), can injected into the mouse brain and released after MMP-9 cleavage.
Self-assembled PA-hyaluronic acid membranes containing MMP-1 clementable epitopes (GPQGIWGQ) allow the study of cell-ECM remodeling interactions.
A number of MMP-sensitive peptides have optimized to ensure high enzyme sensitivity and specificity, including sequences from phage display libraries, such as SGESPAYYTA (optimized for MMP-2), GAPFALRLV (optimized for MMP-3 and MMP-7), and GAPFALRLV (optimized for MMP-3 and MMP-7).
GGYAELRMGG (optimized for MMP-11) and GPLGLWAR (optimized for MMP-13), GPQGIAGQ (sensitive to MMP-1, -2, -3, -7, -8, and -9), GPLGMRGL (can cut by MMP-13), And the sequences VPMSMRGG and IPESLRAG.
They have a high degradation rate of MMP-1 and MMP-2. Mmp-sensitive epitopes have also used in dynamic matrix design to induce morphogenesis and enhance cell invasion.
For example, the realization of MMP-sensitive GPQGIWGQ epitopes in PEG hydrogels could enable proteolytic remodeling and organogenesis of intestinal stem cells in vitro.
Similarly, renal tubule formation can achieved in vitro by modulating the mechanism and degradability of heparin-based hydrogels containing MMP-sensitive GPQGIWGQ sequences.
Alginate gels modified with MMP-sensitive PVGLIG epitopes deliver human mesenchymal stem cells and ensure cell invasion and subsequent degradation after subcutaneous implantation in mouse models.
b. Peptides cleaved by other enzymes
In addition to MMPs, other enzymes have explored to make degradable peptide-based hydrogels;
For example, a PAs showing the consistent sequence RRX1SX2, where X1 can be any residue and X2 must be a hydrophobic residue that is sensitive to cleavage of protein kinase a (PKA).
Therefore, PA nanofibers that display RRASV assembled and disassembled according to specific enzymes.
When exposed to PKA, the nanofibers phosphorylated and broken down, while in the presence of ALP, the phosphate groups split, restoring the system’s assembly capacity.
The serine protease alpha-chymotrypsin cleaves the KKFFVLK sequence next to the F residue, resulting in different products that tend to form nanotubes or spherical micelles.
Similarly, cathepsin sensitive sequences can used to create degradable substrates;
For example, in bone absorption studies using porcine cortical bone slices as substrates, PEGDA hydrogels grafted with the cathepsin K-sensitive peptide GGGMGPSGPWGGK showed specific degradation of osteoclasts but no response to osteoblasts.
The cathepin-B cleavable peptide AVPIAQFRRG can coupled with pro-apoptotic peptide drugs and doxorubicin to achieve high cancer cell specificity in tumor-bearing mice.
In addition, by adding ester skeletons to the β-sheet nanofibers and gels, they can degraded by hydrolysis.
Therefore, degradation can made using specific peptides and customized by changing their secondary structure or amino acid composition to create dynamic biomaterials that are conducive to cell remodeling and cell response to matrix turnover.

Hydrogels: have a synergistic peptide motif
a. Mixture of cell adhesion epitopes
ECM components usually work together;
For example, fibronectin contains an RGDS ring and a pentapeptide PHSRN, which are close to each other on the folded protein and can synergistically bind α5β1 integrin when they are 3.2 nm apart.
Thus, a multi-domain peptide system displaying RGDS and PHSRN at approximately 3.2 nm between epitopes can control endothelial cell adhesion and integrin upregulation in vitro.
Multiple peptide epitopes can incorporated into the same hydrogel by co-assembling different epitopes display chains.
For example, Q11 carrying RGDS, IKVAV, REDV, and YIGSR can combined in different combinations to form homogeneous gels with different mechanical and biological properties depending on the concentration of peptide epitopes mixed.
Interestingly, RGDS and YIGSR exhibit antagonist effects, while RGDS and IKVAV exhibit additive synergies.
RGD and IKVAV can mixed for cardiac tissue engineering, IKVAV and YIGSR can mixed for neurogenesis, and RGDS and phosphoserine can mixed for bone regeneration research.
b. Mixture of cell adhesion and growth factor binding epitopes
Cell adhesion peptides can also bind to growth factor binding epitopes.
For example, polyacrylates can unfurl fibronectin by adsorbing and assembling proteins on methyl groups of polyacrylate monomers, thereby causing conformational changes in fibronectin and exposing its epitopes (FNIII9-10 and FNIII12-14), enabling synergistic adhesion and growth factor signaling 73.
Recombinant fibronectin functionalized 3D fibrin hydrogels containing two bioactive fragments (FNIII9-10 and FNIII12-14) loaded with VEGF to promote wound healing in diabetic mice.
The same platform can loaded with BMP-2 for bone regeneration of bone defects.
Polyethylene glycol hydrogels containing full-length fibronectin have adjustable physical properties that retain BMP-2 and VEGF to aid bone regeneration and blood vessel formation in the body.
rad16-1 can bind to fibra-nexin and lamin-derived motif and heparin-binding sequence TAGSCLRKFSTM in cultured hepatocytes.
Synthetic stem cell niches can designed using rada16-1, which functionalized by SKPPGTSS (bone marrow homing motif), FHRRIKA (heparin-binding motif), and PRGDSGYRGDS (two-unit RGDS cell adhesion motif).
RADA16 hydrogel can modified with epitopes derived from RGD and vegf to promote dentin-pulp regeneration, and EAK16-II hydrogel can functionalized with fibronectin RGD and (GRGDSP) 4K, vitreous nexin FRHRNRKGY, laminin IKVAV and IGF-1. For neural tissue engineering.
The hydrogel containing the bdnf analog motif and IKVAV sequence can used as the filling of the empty chitosan tube to bridge the 10 mm long sciatic nerve defect in rats, thereby achieving peripheral nerve regeneration.
Multiple epitope methods can also used to stimulate osteogenesis and bone formation.
For example, ELP-based membranes containing REDV, RGDS, and Statherin-derived peptide DDDEEKFLRRIGRFG improved endothelialization, cell adhesion, and calcium phosphate binding, respectively, resulting in increased osteoblast differentiation in vitro and bone deposition in critical-size rat skull defect models.
Similarly, in co-culture with endothelial cells and MSCs, co-assembled PA hydrogels (including RGDS for cell adhesion, SVVYGLR for angiogenesis, and DGEA for osteoblast differentiation) promoted the growth of osteoblasts
c. Mixture of simulated epitopes of multiple growth factors
The results showed that the epitopes of various growth factors could integrated into the same hydrogel.
For example, the combination of CTDIKGKCTGACDGKQC (simulated NGF) and RGIDKRHWNSQ (simulated BDNF) peptides can act as a nerve vascular filling agent to synergically promote the regeneration of peripheral nerve of 10mm sciatic nerve defect in rats.
Similarly, peptides that mimic bdnf can combined with a motif that mimics vegf in a RadA16-based hydrogel to promote peripheral nerve regeneration and bridge in the sciatic nerve space in rats.
In addition to its angiogenic properties, VEGF also has neurotrophic and neuroprotective effects.
Thus, rat Schwann cells cultured on BDNF/ vegf functionalized hydrogels exhibit markers of adhesion, diffusion, and myelination.
In addition, the functional hydrogels can also induce the germination of myelinated fibers, the regeneration of axons and the recovery of motor function in vivo
d. Mixtures of cell adhesion, simulated growth factors, and enzymes that can cut epitopes
Matrix dynamics, cell adhesion, and growth factor signaling can also realized within the same system.
For example, the multi-domain peptide system “SLanc” contains the cell adhesion sequence RGDS, an MMP-2 sensitive peptide, and a VEGF-mimicking peptide.
Subcutaneous injection of slac hydrogel in rats promoted the formation of a large number of blood vessels around the implant within 7 days, with rapid host cell infiltration and insufficient fiber embedding 84.
RGD can also bind to mmp-2 sensitive peptide sequences in a hydrogel carrier for delivery of mesenchymal stem cells to increase mesenchymal stem cell attachment and cell remodeling of scaffolds.
PA systems containing RGD and mmp-2 sensitive cutting sites (TPGPQGIAGQ) enable “self-separation” of tissue templates during cell culture for downstream applications.
A 3D PEG ECM-inspired hydrogel containing 13 cell adhesion integrin-binding peptides and 7 MMP-degradable peptides designed to mimic the diversity of proteins in human bone marrow.
Here, peptides selected from natural tissues by means of proteomic and biomechanics-based methods.
Specific combinations of bone marrow-derived adhesion cues and cleavable sites reproduce the bulk modulus of natural bone marrow and provide a biomimetic niche for MSC growth and differentiation.
Similarly, natural brain peg-based hydrogels containing nine integrin-binding peptides and five MMP-degradable peptides were able to control and maintain astrocyte quiescence in vitro with minimal activation.