Glutathione plays a variety of roles in cell physiology, and clinical studies on the alteration of glutathione homeostasis have received much attention.
The number of disorders associated with impaired glutathione homeostasis continues to increase, reflecting the importance and diversity of glutathione cell function.
The most well-known include neurodegenerative diseases (particularly Parkinson’s and Alzheimer’s diseases), lung diseases (chronic obstructive pulmonary disease, asthma and acute respiratory distress syndrome), cystic fibrosis, immune diseases (HIV, autoimmune diseases), cardiovascular diseases (hypertension, myocardial infarction, cholesterol oxidation), And age-related oxygen-related diseases (such as cataracts, macular degeneration, hearing impairment, and glaucoma), as well as the aging process itself.
Reduced glutathione homeostasis, disease mechanism and diagnosis and treatment
GSH deficiency can lead to the progression of a variety of diseases, including immune deficiency, neuropathy, cancer, and more.
Various vitamins, minerals, organic sulfur compounds, and polyphenols can act as natural antioxidants and increase GSH levels, thus demonstrating the potential to extend life.
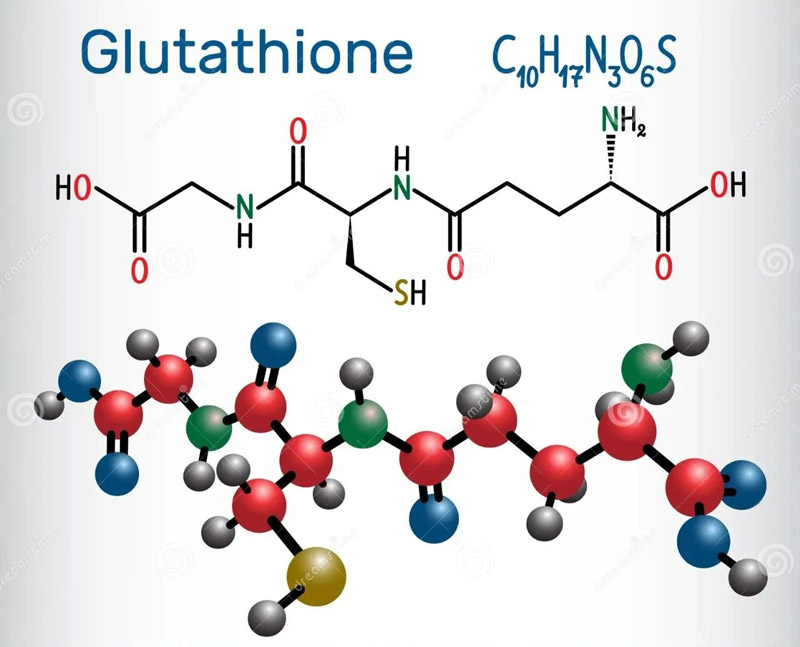
GSH is an intracellular non-protein compound containing sulfhydryl group.
Its high electron donor potential makes it an excellent antioxidant.
Glutathione can assist in the treatment of many diseases, such as cancer, hepatitis, memory loss and heart disease, maintenance of immune responses, and detoxification of heavy metals and drugs.
Injection administration:
GSH can be given orally, sublingual, intravenously, intramuscularly, or even by inhalation.
For patients with lung disease, inhaling GSH(atomizing or nebulizing) is the best option.
GSH can be used for intramuscular injection in chemotherapy patients, HIV-AIDS patients, or male infertility patients.
Intravenous GSH is recommended for anemia patients. GSH protects hepatocytes from reperfusion injury after liver transplantation in rats due to its low toxicity to humans.
Intravenous administration of high doses of NAC as an antioxidant and GSH precursor increases blood and brain GSH levels in patients with Parkinson’s disease.
Oral administration:
Oral administration of GSH is controversial because gut and liver gamma-glutamyltransferase (GGT) metabolize GSH and reduce the bioavailability of GSH given.
Using a sublingual delivery system avoids enzymatic degradation of GSH because GSH is a small peptide that is well absorbed by mucous membranes.
The oral consumption of GSH is related to its bioavailability. By looking at the benefits of oral administration, the researchers developed orally active acetyl GSH, which is also more stable in plasma and the gut.
Glutathione and tumor diagnosis and treatment
Due to the rapid proliferation rate of tumor cells, their metabolic requirements are high.
We further our understanding of the complexity of tumor cell metabolism and REDOX chemistry in cancer etiology, progression, and treatment.
Mitochondrial REDOX reactions are the main source of ROS in most cell types, including tumor cells.
To avoid the toxic effects of ROS on DNA, proteins, and lipids,
cells have evolved a wide range of antioxidant mechanisms, especially in tumor cells with extremely high REDOX reactivity.
Glutathione is now known to outperform its central role in the regulation of reactive oxygen species (ROS).
Many ROS defense mechanisms rely on glutathione molecules.
Tumors increase the burden of reactive nitrogen (RNS). Through its associated reactions and interactions with many proteins, glutathione is one of the main regulators of cancer cell development, progression, response to therapy and their environment, and many therapeutic targeting pathways have been derived based on this mechanism.
REDOX and ROS are also particularly important in tumor cell signaling pathways associated with cancer progression through their roles in tumorigenesis, tumor cell migration, and tumor cell survival.
Compared with healthy cells, tumor cells showed abnormal ROS homeostasis. Most cancer cells have a higher ROS set point than non-cancer cells.
Elevated GSH levels have been observed in many cancer patients, including breast, ovarian, lung, and head and neck cancers.
Through its related physiological functions, glutathione plays an important role in tumors, and many studies have shown that increased cellular levels of glutathione are necessary for tumor genesis and proliferation.
High ROS production in cancer cells requires high activity of the cell’s antioxidant system, making cancer cells allergic to exogenous agents that impair their antioxidant capacity.
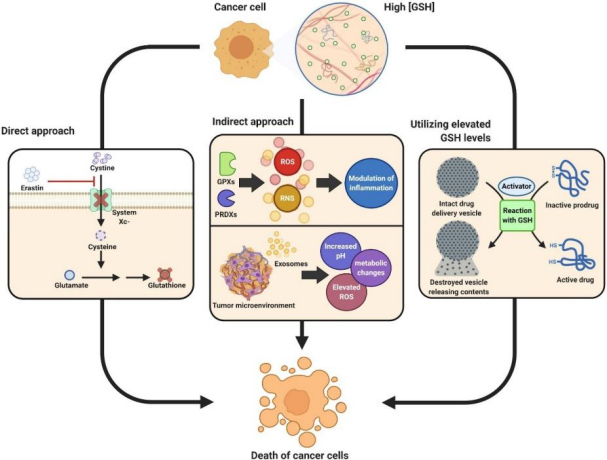
Reducing the production or availability of glutathione provides a new approach for tumor therapy.
Inhibition of the cysteine cell transport system by drugs can lead to GSH depletion and cell death in the form of iron apoptosis.
For example, glutathione depletion induced by the use of the small molecule drug Erastin can significantly increase the cytotoxicity of cisplatin and enhance the clinical effect.
ROS and GSH-responsive nanoparticle drug delivery systems could serve as a way to deliver highly toxic payloads to cancer cells more specifically and safely.
Ling et al. found that GSH-induced disintegration of optimized nanoparticles releases their payload of active platinum Pt (II) metabolites, which then covalently bind to target DNA and induce apoptosis in cancer cells.
Therapeutic effect of glutathione on liver disease
In the liver, glutathione (GSH) plays a particularly important role as an antioxidant because it is the primary site of liver synthesis, storage, and output.
The importance of glutathione in the liver lies in the organ’s central role in oxidizing and eliminating substances such as ethanol and other toxic products that induce oxidative stress;
In the treatment of viral liver disease, reduced glutathione can enhance the detoxification function of liver through transmethylation and transpropylamino reaction, and promote bile metabolism, thus protecting liver cells.
The liver requires the presence of antioxidants to prevent or reduce this stress by capturing, metabolizing, or converting molecules into agents less toxic than ROS.
In the treatment of drug-induced liver disease, glutathione can effectively reduce the side effects of drugs and protect the liver.
In the treatment of alcoholic liver disease, glutathione can accelerate free radical excretion, reduce liver cell damage, restore liver detoxification function, and promote liver function recovery.
Oxidative stress is a pathophysiological marker of metabolic liver disease.
In this case, overproduction of ROS appears to be associated with impairment of intracellular GSH homeostasis, resulting in reduced GSH levels and its weakened antioxidant and liver protective functions.
Based on these assumptions, GSH, like other types of liver disease, is also used in the treatment of NAFLD.
An earlier study published by Dentico et al. evaluated the effect of a 30-day intravenous or intramuscular injection of high doses of GSH on hepatolysis index in patients with chronic fatty liver disease.
No adverse reactions were reported, and significant reductions in liver tests (specifically transaminase and gamma-glutamyltranspeptidase-GGT) were detected in all treated patients.
The efficacy of GSH treatment was confirmed, and a reduction in malondialdehyde, a marker of liver cell damage, was detected.
A follow-up study by Irie et al. showed that oral glutathione could prevent the progression of NAFLD to non-alcoholic fatty liver (NASH).
Higher levels of oxidative stress were detected in NASH patients compared to NAFLD.
They evaluated the immunohistochemical expression of GSH in pre-treatment biopsies and found that GSH expression was stronger in NAFL than NASH.
These results suggest that NAFLD may progress to NASH due to oxidative stress and demonstrate the potential therapeutic role of GSH in controlling the progression of liver damage.
These preliminary studies suggest that oral GSH has a potential therapeutic effect on NAFLD.
The small sample size, short duration of treatment, no control group, and lack of liver biopsy evaluation after treatment are just some of the limitations of these studies.
More research is needed to elucidate the mechanisms behind the action of GSH, and large-scale trials are needed to confirm the therapeutic role of GSH.

Glutathione dietary supplement and anti-aging
Under Section 201(s) of the Federal Food, Drug, and Cosmetic Act issued by the US Food and Drug Administration (US-FDA), glutathione-based oral dietary supplements are generally not restricted in the United States.
Since free radicals play an important role in the development of age-related diseases, GSH is considered an effective anti-aging drug due to its outstanding antioxidant properties.
Maintaining optimal GSH levels in different tissues is considered an important strategy for preventing oxidative stress-related diseases.
Richie et al. studied the effectiveness of oral GSH supplements in healthy adults.
At both doses, GSH levels in the blood increased after 1, 3, and 6 months (250 or 1,000 mg/ day).
Daily intake of GSH supplements effectively increased body stores and some immune markers.
Clinical studies have shown that nutritional supplements in the form of amino acids, vitamins, minerals and polyphenols can significantly affect circulating GSH, with genetic variants affecting its levels.
Kumar et al. found that supplementation with glycine and NAC extended the lifespan of laboratory animals by correcting GSH deficiency.
The effect of glycine and NAC on ameliorating GSH deficiency and promoting healthy aging is plausible.
A randomized clinical trial showed that supplementation with glycine and NAC(after 2 and 16 weeks of administration) improved many physical functions and aging characteristics in older adults.
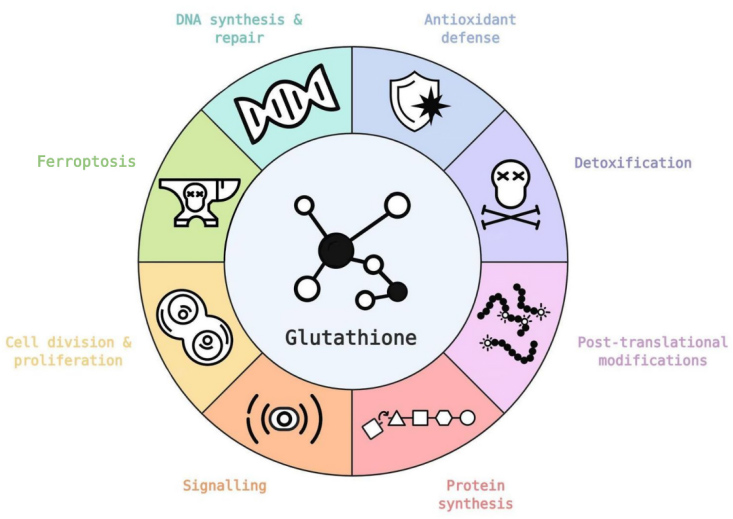
Glutathione plays a very dynamic physiological role in most living cells where it acts directly as an antioxidant and is also a key part (cofactor) of many antioxidant enzymes that inhibit free radicals and overcome oxidative stress.
GSH acts as a regulatory molecule for a variety of non-enzymatic substances, such as vitamins or polyphenols, to prevent ROS damage.
Glutathione produces some metabolites that can enter the Krebs cycle to produce energy for the body.
Due to the extensive and important biochemical characteristics of glutathione, its medicinal range is expanding day by day, and many domestic and foreign studies have shown the effectiveness and safety of GSH in the treatment of some diseases, proving that GSH, as an antioxidant and cell metabolism regulator,
is an important therapeutic or auxiliary therapeutic drug in multi-disciplines and multi-diseases, especially in the rescue of severe diseases.
The clinical and experimental research of glutathione in some fields needs to be further strengthened.
reference
- [1]Gasmi A, Nasreen A, Larysa Lenchyk L, et al, An Update on Glutathione’s Biosynthesis, Metabolism, Functions, and Medicinal Purposes, Current Medicinal Chemistry, Pub Date: 2023.
- [2]Varesi, A.; Campagnoli, L.; Pierella, E.; Bavestrello Picci- ni, G.; Carrara, A.; Ricevuti, G.; Scassellati, C.; Bonvicini, C.; Pascale, A. The Role of Antioxidants in the Interplay between Oxidative Stress and Senescence; Antioxidants: Basel, Switzerland, 2022: 11.
- [3]Gasmi, A.; Shanaida, M.; Oleshchuk, O.; Semenova, Y.; Mujawdiya, P.K.; Ivankiv, Y.; Pokryshko, O.; Noor, S.; Piscopo, S.; Adamiv, S.; Bjørklund, G. Natural ingredients to improve immunity. Pharmaceuticals., 2023, 16(4):528.
- [4]Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids, 2012: 1-26.
- [5]Witschi, A.; Reddy, S.; Stofer, B.; Lauterburg, B.H. The systemic availability of oral glutathione. Eur. J. Clin. Pharmacol., 1992, 43(6):667-669.
- [6]Prousky, J. The treatment of pulmonary diseases and respiratory-related conditions with inhaled (nebulized or aerosolized) glutathione. Evid. Based Complement. Alternat. Med., 2008, 5(1): 27-35.
- [7]Palamara, A.T.; Garaci, E.; Rotilio, G.; Ciriolo, M.R.; Cas- ablanca, A.; Fraternale, A.; Rossi, L.; Schiavano, G.F.; Chiarantlni, L.; Magnani, M. Inhibition of murine AIDS by re- duced glutathione. AIDS Res. Hum. Retroviruses, 1996, 12(14):1373-1381.
- [8] Coles, L.D.; Tuite, P.J.; Öz, G.; Mishra, U.R.; Kartha, R.V.; Sullivan, K.M.; Cloyd, J.C.; Terpstra, M. Repeated-dose oral n-acetylcysteine in Parkinson’s disease: Pharmacokinetics and effect on brain glutathione and oxidative stress. J. Clin. Pharmacol., 2018, 58(2): 158-167.
- [9] Schmitt, B.; Vicenzi, M.; Garrel, C.; Denis, F.M. Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: A comparative crossover study. Redox Biol., 2015, 6:198-205.
- [10] Kretzschmar, M. Regulation of hepatic glutathione metabolism and its role in hepatotoxicity. Exp. Toxicol. Pathol., 1996, 48(5): 439-446.
- [11] Kennedy L, Sandhu J K, Harper M-E, Cuperlovic-Culf M, Role of Glutathione in Cancer: From Mechanisms to Therapies, Biomolecules 2020, 10: 1429.
- [12] Luanpitpong, S.; Talbott, S.J.; Rojanasakul, Y.; Nimmannit, U.; Pongrakhananon, V.; Wang, L.; Chanvorachote, P. Regulation of Lung Cancer Cell Migration and Invasion by Reactive Oxygen Species and Caveolin‐1. J. Biol. Chem. 2010, 285: 38832–38840.
- [13] Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione levels in human tumors. Biomarkers, 2012, 17:671–691.
- [14] Harris, I.S.; Treloar, A.E.; Inoue, S.; Sasaki, M.; Gorrini, C.; Lee, K.C.; Yung, K.Y.; Brenner, D.; Knobbe‐Thomsen, C.B.; Cox, M.A.; et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 2015, 27: 211–222.
- [15] Cramer, S.L.; Saha, A.; Liu, J.; Tadi, S.; Tiziani, S.; Yan, W.; Triplett, K.; Lamb, C.; Alters, S.E.; Rowlinson, S.; et al. Systemic depletion of L‐cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat. Med. 2017, 23:120–127.
- [16] Kennedy L, Sandhu J K, Harper M-E, Cuperlovic-Culf M, Role of Glutathione in Cancer: From Mechanisms to Therapies, Biomolecules 2020, 10:1429.
- [17] Sato, M.; Kusumi, R.; Hamashima, S.; Kobayashi, S.; Sasaki, S.; Komiyama, Y.; Izumikawa, T.; Conrad, M.; Bannai, S.; Sato, H. The ferroptosis inducer erastin irreversibly inhibits system x‐ and synergizes with cisplatin to increase cisplatin’s cytotoxicity in cancer cells. Sci Rep. 2018, 8: 968.
- [18] Chen, D.; Zhang, G.; Li, R.; Guan, M.; Wang, X.; Zou, T.; Zhang, Y.; Wang, C.; Shu, C.; Hong, H.; et al. Biodegradable, Hydrogen Peroxide, and Glutathione Dual Responsive Nanoparticles for Potential Programmable Paclitaxel Release. J. Am. Chem. Soc. 2018, 140: 7373–7376.
- [19] Ling, X.; Tu, J.; Wang, J.; Shajii, A.; Kong, N.; Feng, C.; Zhang, Y.; Yu, M.; Xie, T.; Bharwani, Z.; et al. Glutathione‐Responsive Prodrug Nanoparticles for Effective Drug Delivery and Cancer Therapy. ACS Nano 2018, 13: 357–370.
- [20] Lu Xiaobi. Clinical observation of reduced glutathione in the treatment of chronic viral hepatitis complicated with alcoholic liver injury. Journal of Clinical Rational Drug Use, 2019, 12(5): 15-16,20.
- [21] Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014, 20: 8082–91.
- [22] Sacco R, Eggenhoffner R, Giacomelli L. Glutathione in the treatment of liver diseases: insights from clinical practice. Minerva Gastroenterol Dietol. 2016, 62: 316–24.
- [23] Massa P, Coccia G, et al. Reduced gluthatione in alcoholic hepatophaty. Giornale Ital Ricerche Clin Ter. 1993, 14: 87–92.
- [24] Dentico P, Volpe A, Buongiorno R, Grattagliano I, Altomare E, Tantimonaco G, et al. Il glutatione nella terapia delle epatopatie croniche steatosiche [glutathione in the treatment of chronic fatty liver diseases]. Recenti Prog Med. 1995, 86: 290–3.
- [25] Irie M, Sohda T, Anan A, Fukunaga A, Takata K, Tanaka T, et al. Reduced glutathione suppresses oxidative stress in nonalcoholic fatty liver disease. Eur J Hepatogastroenterol. 2016, 6: 13–8.
- [26] Homma, T.; Fujii, J. Application of glutathione as anti- oxidative and anti-aging drugs. Curr. Drug Metab., 2015, 16(7): 560-571.
- [27] Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della- Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bona- duce, D.; Abete, P. Oxidative stress, aging, and diseases. Clin. Interv. Aging, 2018, 13: 757-772.
- [28] Richie, J.P., Jr; Nichenametla, S.; Neidig, W.; Calcagnotto, A.; Haley, J.S.; Schell, T.D.; Muscat, J.E. Randomized con- trolled trial of oral glutathione supplementation on body stores of glutathione. Eur. J. Nutr., 2015, 54(2): 251-263.
- [29] Minich, D.M.; Brown, B.I. A review of dietary (Phyto)nutrients for glutathione support. Nutrients., 2019, 11(9): 2073.
- [30] Kumar, P.; Osahon, O.W.; Sekhar, R.V. GlyNAC (Glycine and N-Acetylcysteine) supplementation in mice increases length of life by correcting glutathione deficiency, oxidative stress, mitochondrial dysfunction, abnormalities in mitophagy and nutrient sensing, and genomic damage. Nutrients., 2022, 14(5): 1114.
- [31] Sekhar, R.V. GlyNAC supplementation improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, aging hallmarks, metabolic defects, muscle strength, cognitive decline, and body composition: Implications for healthy aging. J. Nutr., 2021, 151(12):3606-3616.
- [32] Kumar, P.; Liu, C.; Suliburk, J.; Hsu, J.W.; Muthupillai, R.; Jahoor, F.; Minard, C.G.; Taffet, G.E.; Sekhar, R.V. Supplementing Glycine and N-Acetylcysteine (GlyNAC) in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, physical function, and aging hallmarks: A randomized clinical trial.J. Gerontol. A Biol. Sci. Med. Sci., 2023, 78(1): 75-89.