Key research points
Glutathione is an important tripeptide that is widely used in the pharmaceutical, food and cosmetic industries.
In this paper, metabolic engineering of Escherichia coli (E. coli) was used to produce glutathione efficiently.
Conventional glutathione is mainly produced by yeast fermentation, but the intracellular accumulation of yeast limits its production.
The researchers significantly increased glutathione production through overexpression of key enzyme coding genes gshA and gshB, as well as through a series of gene knockouts and metabolic pathway optimization.
In particular, through continuous feed fermentation and the use of sodium thiosulfate as a sulfur source, a glutathione yield of 22.0 g/L was finally achieved, which is the highest yield reported in the current literature.
This study provides a new and efficient method for industrial production of glutathione.

Genetic engineering:
The glutathione biosynthesis pathway was enhanced through overexpression of gshA and gshB genes (encoding cysteine glutamate ligase and glutathione synthase, respectively).
Gene knockout strategy:
ggt, pepT and gor genes (encoding glutathione oxidoreductase) were knocked out, reducing glutathione degradation and GSSG generation, and increasing total glutathione production.
Optimization of secretion pathway:
By knocking out the yliABCD gene, the reuptake of glutathione was reduced and its secretion was promoted.
Innovative use of sulfur source:
The use of sodium thiosulfate instead of sulfate as sulfur source reduces NADPH consumption and improves cysteine production efficiency.
Continuous feed fermentation:
Efficient production of glutathione achieved through continuous feed glycine and sulfur supplements.
High yield record:
A glutathione yield of 22.0 g/L was achieved, which, to the authors’ knowledge, the highest yield currently reported in the literature.
No need to add cysteine:
Unlike previous studies, this study did not add expensive cysteine in the production process, reducing production costs.
Metabolic flux and metabolome analysis:
A combination of metabolic flux analysis (MFA) and metabolome analysis accurately identifies and addresses rate-limiting steps in the production process.
Environmentally friendly production:
Since glutathione secreted into the medium, the downstream purification process is simplified and more environmentally friendly.
Chart interpretation
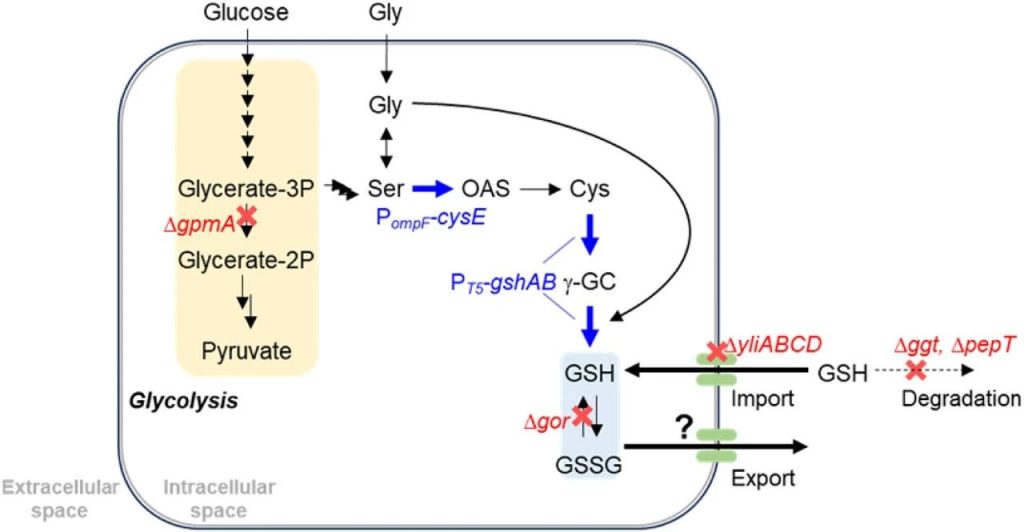
Figure 1: Schematic diagram of glutathione biosynthesis in E. coli.
The metabolic pathway from glucose to cysteine described, including through the conversion of Glycerate 3-phosphate, serine, and O-acetylserine.
The process by which cysteine binds to glutamate and glycine to produce reduced glutathione (GSH) shown, and GSH secreted by an unknown outlet protein.
Strategies for genetic modification highlighted, including knocked out genes (red) and overexpressed genes (blue).
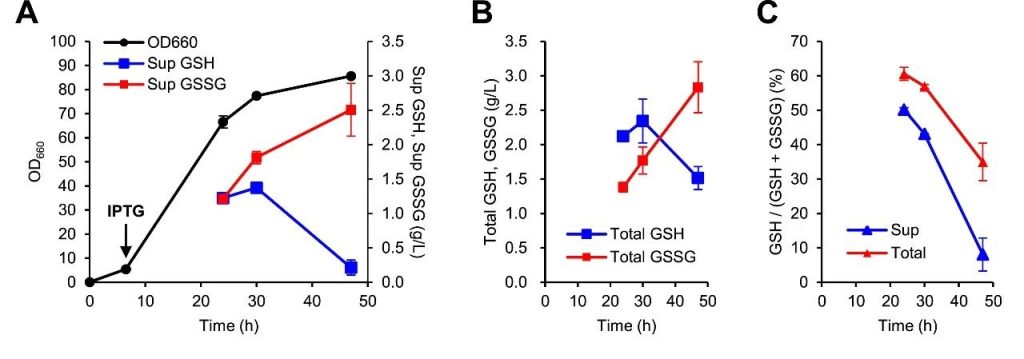
Figure 2: Proof of concept for glutathione fermentation of KG0A strain in a 5L bioreactor.
(A) showed cell growth (OD660 value) and concentrations of GSH and oxidized glutathione (GSSG) in the supernatant.
(B) Showed the total amount of GSH and GSSG after 47 hours of culture.
(C) Demonstrated the ratio of GSH to total glutathione (GSH + GSSG) in the supernatant over time.
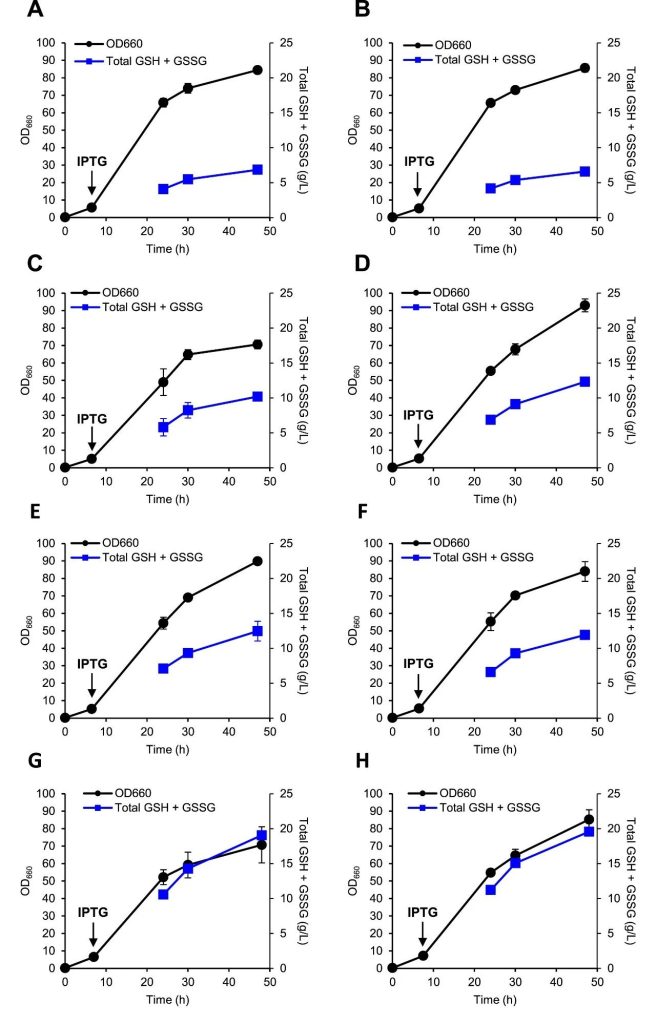
Figure 3: Genetic engineering to increase glutathione production.
The cell growth (OD660 value) and total GSH and GSSG concentrations of different engineered strains (KG0B, KG0C, KG0D, KG01, KG02, KG04, KG05, KG06) demonstrated.
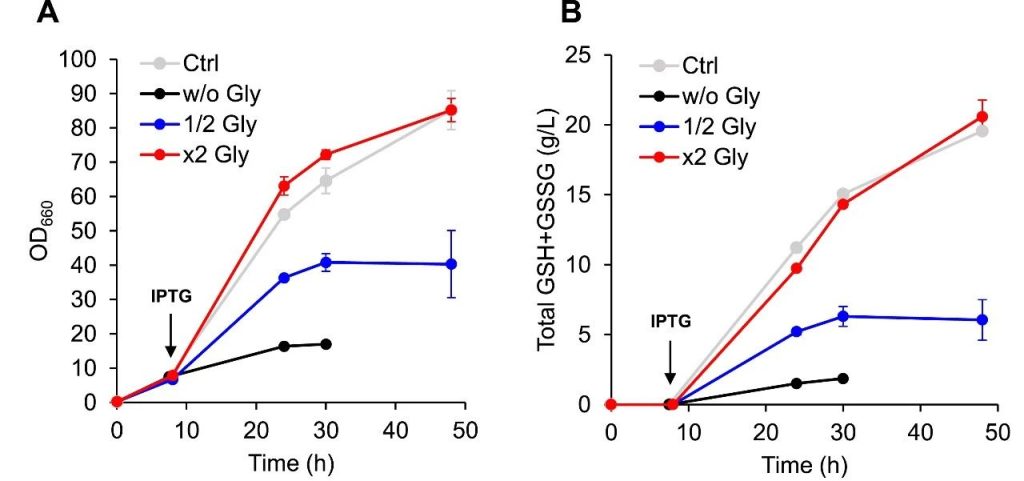
Figure 4: The key role of glycine in glutathione fermentation.
The cell growth (OD660 value) and the changes of GSH and GSSG concentration of KG06 strain under different concentration of glycine supplement demonstrated.
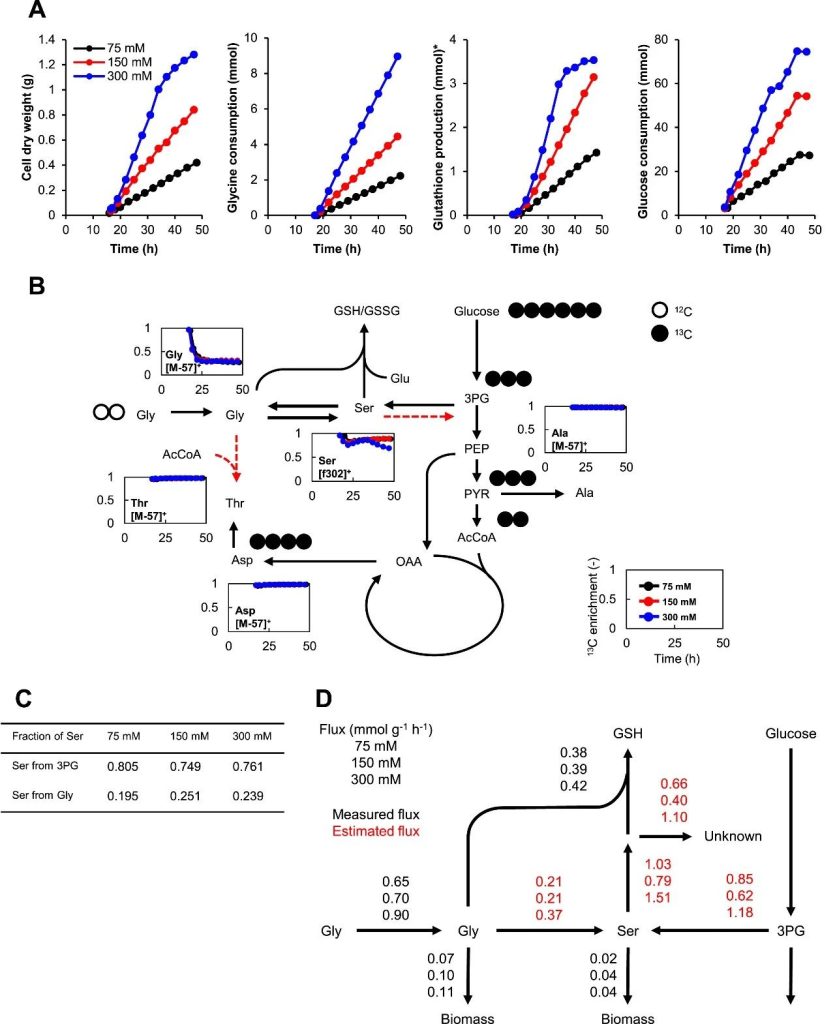
Figure 5: KG06 cultured in a 200mL bioreactor for metabolic flux analysis (MFA) using [U-13C] D-glucose and three different concentrations of unlabeled glycine.
(A) Biomass production, glycine consumption, glutathione production and glucose consumption shown.
(B) 13C enrichment data for protein-derived amino acids are presented.
(C) Demonstrated the source of protein-derived serine.
(D) Demonstrated the flux distribution in the glutathione synthesis pathway.
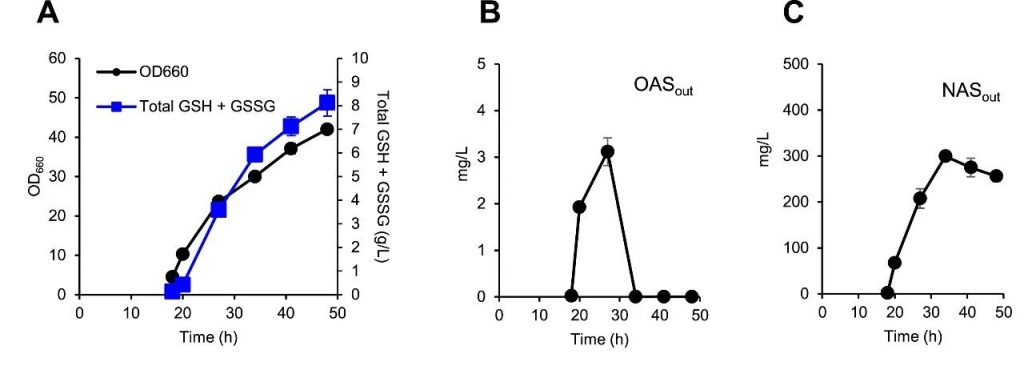
Figure 6: Metabolome analysis of KG06 in a 200 mL bioreactor.
(A) Cell growth (OD660 value) and total concentrations of GSH and GSSG.
(B) Extracellular O-acetylserine (OAS) concentration.
(C) Extracellular concentrations of N-acetyl-L-serine (NAS).
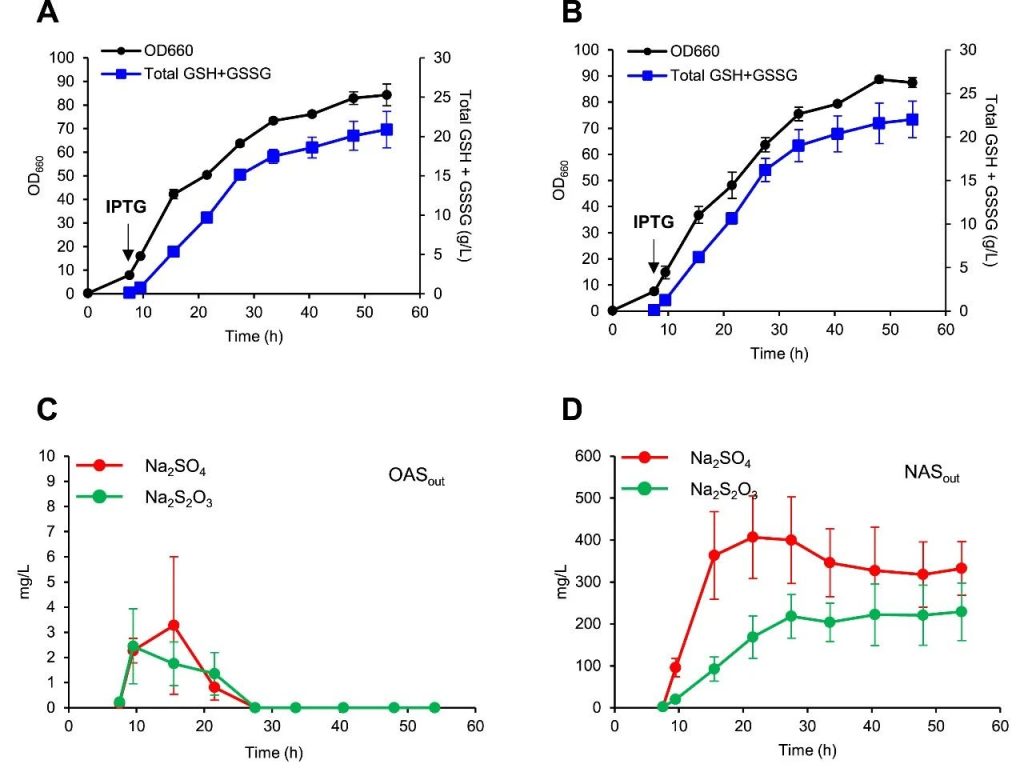
Figure 7: KG06 cultured using sodium sulfate (A) or sodium thiosulfate (B) in a 5 L bioreactor.
Cell growth (OD660 values) and GSH and GSSG concentrations shown.
(C) Extracellular OAS concentration.
(D) Extracellular NAS concentration.
Enlightenment and reflection
Understanding of biosynthetic pathways:
A deep understanding of the dynamics and regulatory mechanisms of the individual steps in the biosynthetic pathway is essential for designing effective metabolic engineering strategies.
Identification of rate-limiting steps:
The rate-limiting steps identified through metabolic flux analysis and metabolome analysis provide clear directions for further increase in yield.
Sulfur source selection:
The use of sodium thiosulfate instead of sulfate as the sulfur source in this paper improves the production efficiency of cysteine, which shows that in industrial production, the selection of suitable precursors and cofactors can significantly affect the production efficiency.
Environmentally friendly production:
The glutathione achieved in the study secreted into the medium, reducing downstream purification steps and providing a new idea for environmentally friendly production.
Further metabolic pathway optimization:
Although remarkable results have achieved in this paper, there may other rate-limiting steps or bottlenecks in the metabolic pathway that need to further explored and optimized.
Dynamic regulatory strategies:
Develop dynamic regulatory strategies, such as the use of sensors to control gene expression, to achieve more precise metabolic control.
Diversity of host strains:
Explore other types of microbial hosts to determine if they can provide additional advantages, such as higher yields or lower production costs.
Glutathione concept learning
Rate-Limiting Step:
Rate-limiting step refers to the slowest step in a series of biochemical reactions, which determines the rate of the entire metabolic pathway.
Through metabolic flux analysis and metabolome analysis, the conversion of o-acetylserine to cysteine identified as the rate-limiting step in the glutathione synthesis pathway.
Understanding and modifying rate-limiting steps is the key to improving the efficiency of biosynthesis.
Metabolic Flux Analysis (MFA) :
MFA a quantitative assay used to study the rate of substance transformation in intracellular metabolic pathways.
With isotopically labeled substrates, it is possible to trace the carbon flow of metabolites and thus determine the activity of individual metabolic pathways.
In this paper, MFA used to identify rate-limiting steps in the glutathione synthesis pathway and evaluate the effect of modification strategies.
Metabolomic Analysis:
Metabolome analysis is a branch of systems biology that focuses on a comprehensive analysis of the composition and changes of all small molecule metabolites in an organism under specific conditions.
In this study, metabolome analysis used to monitor the dynamic changes of key metabolites, revealing patterns of accumulation and consumption during glutathione biosynthesis.
Cysteine:
Cysteine is a sulfur-containing amino acid that is essential for a variety of physiological functions in living organisms, including as a component of GSH. The biosynthesis of cysteine involves complex regulatory mechanisms and is a key precursor for glutathione production.
In this paper, glutathione increased by enhancing cysteine production.
Sulfur Source:
Sulfur source is necessary for biosynthesis of sulfur-containing amino acids such as cysteine and methionine.
In this paper, sodium thiosulfate used as the sulfur source, which consumes less NADPH when reduced to hydrogen sulfide than sulfate, thus supporting cysteine synthesis more efficiently.
Continuous Fed-Batch Fermentation:
Continuous feed fermentation a fermentation technique in which nutrients continuously added during fermentation to maintain cell growth and product synthesis.
In this paper, a high yield of glutathione achieved through continuous supplementation of glycine and sulfur.
Industrial applications of glutathione:
Because of its antioxidant properties, glutathione widely used in the pharmaceutical, food additive and cosmetic industries.
In medicine, it used to treat certain diseases and as a health supplement; In the food industry, as a natural preservative; In cosmetics, used in anti-aging products.
This study provides a new, cost-effective glutathione production method for these applications.