Universities jointly use engineered yeast to break through the production bottleneck of Brazil’s green propolis active ingredient, and record production of atipyrine C
Brazilian green propolis is a natural product with rich medicinal value, and its main active ingredient, Artepillin C, has been confirmed in a number of studies with anti-inflammatory, antioxidant, antibacterial and anti-cancer pharmacological effects.
It has also been found to able to improve obesity and diabetes by activating key regulators of adipocyte differentiation.
However, the production of atipyrine C has faced great challenges.
Its mainly derived from Baccharis dracunculifolia, a plant unique to Brazil. The distribution area of this plant is narrow, the yield is very low, and the influence of environmental factors makes the content of atipilin C extracted extremely unstable.
The scarcity of resources and the high extraction cost seriously restrict its commercialization and wide application.
Although the method of chemical synthesis of atipyrine C is feasible, the steps are complicated and the economic cost is high, and it is difficult to meet the demand for green production.
To solve this dilemma, synthetic biology technology offers a new solution.
Through gene editing and metabolic engineering, scientists are able to “implant” complex natural product production pathways into microbes, enabling efficient and sustainable biosynthesis.
Based on this philosophy, the research team at Kyoto University and Kobe University selected Pichia Pastoris (Komagataella phaffii), which has excellent metabolic potential, and finally achieved the efficient biosynthesis of atipyrine C by introducing key enzymes and optimizing metabolic pathways.
This study not only set a new record for the production of atipyrine C, but also set a new benchmark for the application of synthetic biology in the production of complex natural products.
The work published in ACS Synthetic Biology, Entitled “De Novo Production of the Bioactive Phenylpropanoid Artepillin C Using Membrane-Bound Prenyltransferase in. Komagataella phaffii “.
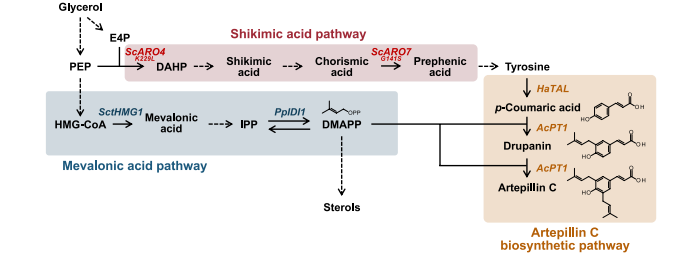
The research team first constructed a synthetic pathway for atipyrine C.
The plant enzyme AcPT1 (coumaric acid-specific dipentenyltransferase), which is essential for the production of atipyrine C, was discovered by Kazufum Yazaki of Kyoto University.
The researchers introduced the gene encoding the enzyme into Pichia Pastoris, which is better able to produce components of such chemicals, can grow at higher cell densities and is less prone to accumulating ethanol than brewer’s yeast.
The formation of artipyrine C depends on the binding of p-hydroxycinnamic acid (coumaric acid, p-CA) to two isopentenyl pyrophosphate (DMAPP) molecules, so the key to the study is to effectively integrate the coumarin-producing pathway and the DMAPP producing pathway in Pichia Pastoris.
In the coumarin production pathway, the research team introduced tyrosine ammonia lyase (TAL) to convert tyrosine into coumaric acid (p-CA), and by optimizing the metabolic capacity of Pichia Pastoris, the intracellular accumulation of coumaric acid reached 11 mg/L, much higher than the level of saccharomyces cerevisiae only 0.1 mg/L.
This suggests that Pichia has significant advantages in metabolic shunting and substrate accumulation, and is particularly suitable as a chassis cell for complex metabolite synthesis.
The production of DMAPP depends on the mevalonate (MVA) metabolic pathway of Pichia Pastoris.
To integrate coumaric acid and DMAPP pathways, the research team introduced a plant-derived coumaric acid-specific dipententransferase (AcPT1).
The enzyme has unique catalytic properties, which can catalyze the continuous combination of coumaric acid and two DMAPP molecules to produce atipyrine C.
In order to optimize the expression and function of the enzyme, they also tried to remove the transport peptide sequence of AcPT1, and the results showed that this operation had no significant effect on the yield of atipilin C, indicating that Pichia Pastoris had good compatibility for heterologous enzyme expression.
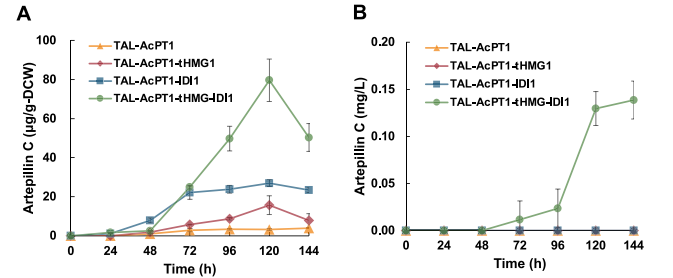
Through the preliminarily constructed pathway, the yield of atipyrine C remained low, at only 4 μg/g-DCW, indicating a metabolic bottleneck requiring further optimization.
To increase the supply of coumaric acid, the research team targeted its upstream metabolic bottleneck with precise modifications. They identified two key enzymes: ARO4 and ARO7.
Normally, these two enzymes inhibited by tyrosine feedback respectively, limiting metabolic flow.
Mutants ARO4K299L and ARO7G141S, which insensitive to tyrosine feedback, were obtained by mutating ARO4 and ARO7 and overexpressed in Pichia Pastoris.
The experimental results showed that the intracellular accumulation of coumaric acid in the modified strain increased significantly, reaching 69 mg/L, which was more than 6 times higher than that of the original strain.
The production of antipyrine C decreased slightly from 4 μg/g-DCW to 3.9 μg/g-DCW, suggesting that coumarin supply is no longer a limiting factor and that other metabolic bottlenecks, such as DMAPP supply, may exist.
Based on this finding, the research team turned the optimization focus to the generation path of DMApps.
They adopted a strategy of overexpression of truncated HMG-CoA reductase (tHMG1) and overexpression of isoprene diphosphate isomerase (IDI1) to enhance the production of DMAPP.
The optimized strain produced 80 μg/g-DCW of atipyrine C, which was 24 times higher than the original strain.
The secretion of atipyrine C detected in culture medium for the first time, and its concentration reached 0.14 mg/L.
In order to solve the problem of further increase in production, the research team adopted a batch feeding culture strategy.
This method supports the high density growth of yeast through continuous supplementation of nutrients.
Under optimized culture conditions, the cell dry weight of yeast increased significantly to 75 g/L, which was much higher than 30 g/L under normal culture conditions.
With the increase of cell dry weight, the production of atipyrine C further increased.
The analysis of intermediate products showed that coumarin supply gradually became a limiting factor under high density culture conditions, which provided an important clue for the next optimization work.
To overcome this bottleneck, the research team adopted a strategy of simultaneously enhancing the supply of DMAPP and coumaric acid.
Building on the already enhanced DMAPP supply, they further introduced ARO4K299L and ARO7G141S mutants to boost coumaric acid production.
Under the optimized batch feeding condition, the metabolic capacity of the strain reached the best state.
The optimized results showed that the accumulation of coumaric acid inside and outside the cell significantly increased, and supply was no longer a limiting factor.
More significantly, the intracellular accumulation of atipyrine C set a new record, eventually reaching 1200 μg/DCW, and the total intracellular and extracellular production reached 12.5 mg/L, which the highest value reported so far.
In the future, the researchers plan to enhance the expression of atipyrine C in two ways.
One approach is to further improve the efficiency of the final and critical chemical steps by modifying the responsible enzyme or increasing the amount of precursor chemicals;
Another approach might be to find a way to transport atipyrine C out of cells.
The implications of this research go far beyond the production of this particular compound.
Since thousands of compounds with very similar chemical structures exist in nature, it is highly likely that the knowledge gained from the production of atipyrine C will applied to the microbial production of other plant-derived compounds.
Reference link:
1. Bamba T, Munakata R, Ushiro Y, et al. De Novo Production of the Bioactive Phenylpropanoid Artepillin C Using Membrane-Bound Prenyltransferase in Komagataella phaffii[J]. ACS Synthetic Biology, 2024, 13(12): 4040-4049.