Glutathione (GSH) is a tripeptide composed of glutamic acid, cysteine and glycine that has a wide range of applications in the pharmaceutical, food and cosmetic industries.
At present, traditional industrial production methods mainly rely on yeast fermentation process to synthesize, but this process has some limitations, GSH will accumulate in yeast cells, and the extraction efficiency is low, limiting its high yield.
Therefore, a new biotechnological method needed to improve the production efficiency and reduce the cost of glutathione.
Previous studies have shown that in the amino acid fermentation process of E. coli,
the product usually secreted from the cell, so as to obtain a high yield, which has certain advantages over yeast.
Engineering Escherichia coli for efficient GSH production by Shingo Kobayashi et al., Nagabuchi, Japan.
The authors used Escherichia coli as chasse to produce glutathione.
Through metabolic engineering and genetic modification,
the biosynthetic pathway of GSH in Escherichia coli enhanced to improve its yield and secretion efficiency.
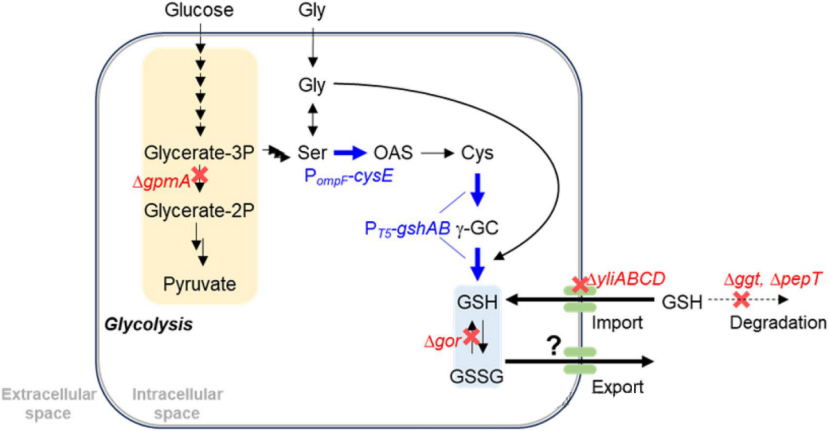
The authors modified Escherichia coli to orthogonally increase glutathione production by the following strategies
Strategy 1: Overexpression of genes encoding key enzymes in glutathione biosynthesis, such as the gshA gene encoding cysteine-glutamate ligase and the gshB gene encoding GSH synthase.
Strategy 2, reduce glutathione degradation. ggt gene encoding gamma-glutamyltransferase and pepT gene encoding tripeptide peptidase deleted.
Strategy 3 promotes the oxidation of GSH. The gor gene encoding glutathione reductase deleted, thus promoting GSH secretion.
Strategy 4: Inhibit cell reuptake of GSH. YliABCD is an ABC transporter involved in the reabsorption of secreted glutathione. The authors deleted the yliABCD gene to reduce the reuptake of GSH by cells and promote its accumulation in the medium.
Strategy 5: Enhance cysteine production. Cysteine is an important precursor of glutathione synthesis, and some studies have shown that CysE is a key enzyme to increase cysteine production. Through overexpression of cysE gene, ompF promoter directly inserted into the upstream of cysE coding sequence, increasing the conversion rate from serine to O-acetylserine.
Strategy 6: Partially block the glycolytic pathway and increase the carbon flux from 3-phosphoglycerate to serine. The authors deleted the gpmA gene encoding phosphoglycerate mutase, partially blocking the glycolytic pathway and shifting more carbon flow from 3-phosphoglycerate to serine, thereby indirectly promoting GSH production.
Through the above series of strategies, the strain that can produce 19.6g/L glutathione finally obtained.
The subsequent authors explored the effect of continuous feeding of glycine on GSH production,
and when the strain cultured without glycine supplementation,
its growth significantly inhibited and only a small amount of GSH produced, suggesting that glycine supplementation essential for glutathione production.
Then, by analyzing the metabolic flow and metabolomics results,
we found that the conversion of O-acetylserine to cysteine is the rate-limiting step for glutathione production, and by using sodium sulfite as the sulfur source,
this limitation largely overcome, and finally the GSH production of the strain increased to 22.0g/L,
which the highest yield reported in the literature so far.
The study of the authors provides a new biotechnological method for industrial-scale production of glutathione.