When we do our research we find that n-acetylcysteine (NAC) is one of the best supplements you can take if your goal is to increase the production of GSH.
If you’ve been around supplements for a while, you’ve no doubt heard of glutathione (GSH), which we call the body’s main antioxidant.
We say Glutathione is the king of antioxidants because it is one of the most abundant and powerful antioxidants in the body, but the best thing about this compound is that the body produces glutathione on its own.
As part of a natural antioxidant defense system. GSH levels are a key factor in overall health. It is extremely difficult to conduct controlled longevity studies in humans, but studies of simple organisms go without saying.
For example, studies of saccharomyces cerevisiae (also known as brewer’s yeast) consistently show that organisms with higher GSH levels live much longer than those with lower GSH levels. In humans, GSH depletion has been linked to the development of serious diseases such as dementia, diabetes, and obesity.
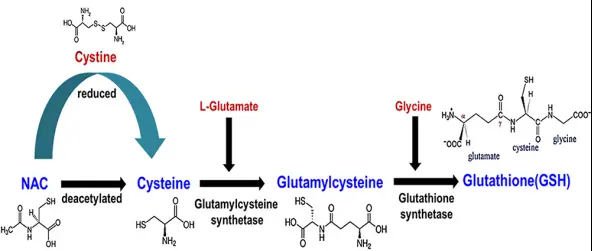
Supplement GSH Precursor (NAC)
Low bioavailability of target molecules is a common problem in supplement science. The best way to solve this problem is usually to take a precursor of the molecule.
Often, their bioavailability is much higher than the target molecule itself.
Fortunately for GSH, this is definitely a viable solution. Studies consistently show that supplementation with GSH precursors, such as cysteine and glycine, can greatly help people with GSH depletion return to normal levels.
Studies on N-acetylcysteine
Once you understand the role of GSH in the body, it’s easy to understand that your health largely depends on keeping the production of GSH at an appropriate level.
Similarly, it is the most powerful and ubiquitous antioxidant in the body, which means it is used in almost all metabolic processes.
Uncontrolled oxidative stress has been linked to rapid aging and the development of major diseases, including cancer, cardiovascular disease, respiratory disease, rheumatoid arthritis, kidney disease, and sexual dysfunction.
There is a two-way relationship between oxidative stress and inflammation. Oxidative stress makes inflammation worse, and inflammation makes oxidative stress worse.
Since chronic systemic inflammation damages basically every tissue in the body, the potentially catastrophic consequences of GSH depletion are obvious.
This, of course, is also the key mechanism behind the link between GSH depletion and metabolic and neurological disorders that we discussed in the introduction.
If you think NAC is too good to be true when you read the following studies, remember that we are dealing with a mechanism that is very fundamental to human health and performance – the management of oxidative stress and inflammation.
This goes a long way to explaining NAC’s extraordinary potential to improve human health.
NAC can improve liver function and detoxification
Let’s first understand why most people seem to be interested in supplementing with NAC – its impact on liver health.
More specifically, interest in NAC has surged in recent years due to the popularity of the fact that NAC can help the body control alcohol toxicity.
It is believed that NAC can promote recovery after drinking while preventing or reducing the intensity of hangovers. This claim is based on animal studies showing that NAC reduces oxidative stress, thereby preventing some of the damaging effects caused by alcohol.
In one study, rats given NAC and alcohol had lower levels of liver enzymes than rats given alcohol alone. Reduced levels of liver enzymes are good because damaged or inflamed liver cells tend to leak their contents into the bloodstream, including these enzymes.
Lower levels of liver enzymes mean less damage and inflammation in liver tissue.
In one particularly interesting study, researchers took a group of mice that showed a preference for alcohol consumption (basically, alcoholic mice) and gave them NAC supplements. When given any amount of alcohol, mice treated with NAC spontaneously drank 65 percent less than mice not supplemented with NAC.
Because of this effect, some researchers have actually proposed that NAC should be used to treat or control alcohol use disorders in humans.
So how exactly does NAC protect the liver from toxic damage? You guessed it – by restoring glutathione levels in liver cells.
In fact, NAC is so effective at protecting the liver from toxins that it’s actually the first-line drug for treating acetaminophen poisoning.
Can NAC fight hangovers? Its effect on acetaldehyde dehydrogenase (ALDH) enzyme
NAC’s ability to strengthen the body’s resistance to the harmful effects of alcohol abuse is partly due to its action on acetaldehyde dehydrogenase (ALDH), an enzyme responsible for detoxifying aldehydes produced when the body metabolizes alcohol.
These aldehyde metabolites are extremely toxic and the sooner they are eliminated, the better.
The good news is that at least one animal study has shown that NAC can significantly up-regulate ALDH activity.
There’s certainly not much research on the topic, so it’s hard to draw firm conclusions right now. But given the positive effects of NAC on overall liver health and function, we wouldn’t be at all surprised to see significant upregulation of ALDH and ADH (alcohol dehydrogenase, the enzyme that converts alcohol into aldehydes).
Neurological benefits of NAC
Because of its ability to promote GSH production, NAC can exert some significant neuroprotective effects.
On the one hand, it helps balance glutamate activity in the brain by providing cysteine, which helps trigger negative feedback on the amount of free glutamate released by neurons.
Excessive glutamate activity can cause neurons to fire uncontrollingly, a condition called excitotoxicity, which is essentially a low-grade neuroinflammation.
Because glutamate-induced excitotoxicity can damage brain tissue and impair cognitive function, it is thought to be a contributing factor to serious mental illness.
According to a meta-analysis and randomized, double-blind, placebo-controlled study, NAC has been shown to improve OCD symptoms.
An animal study suggests that NAC treatment can reverse some of the neurological changes associated with schizophrenia.
It turns out that NAC’s ability to spontaneously reduce alcohol use may also apply to other drugs. A study of 18 – to 21-year-old people of all genders found that after four weeks of NAC supplementation, participants’ daily marijuana use decreased from 15.9 at the start of the study to 11.9 by week four.
That is, by supplementing with NAC alone, cannabis use was reduced by about 25 percent.
To sum up, what is the theme behind it? NAC appears to help stabilize brain function by upregulating GSH. This makes it potentially useful for treating a range of neurological conditions that have traditionally been seen as intractable – as a 2013 editorial advocating for more NAC research noted.
The respiratory benefits of NAC
Another body system affected by GSH production is the respiratory system. Consumption of GSH is associated with the development of respiratory diseases such as chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis, and cystic fibrosis.
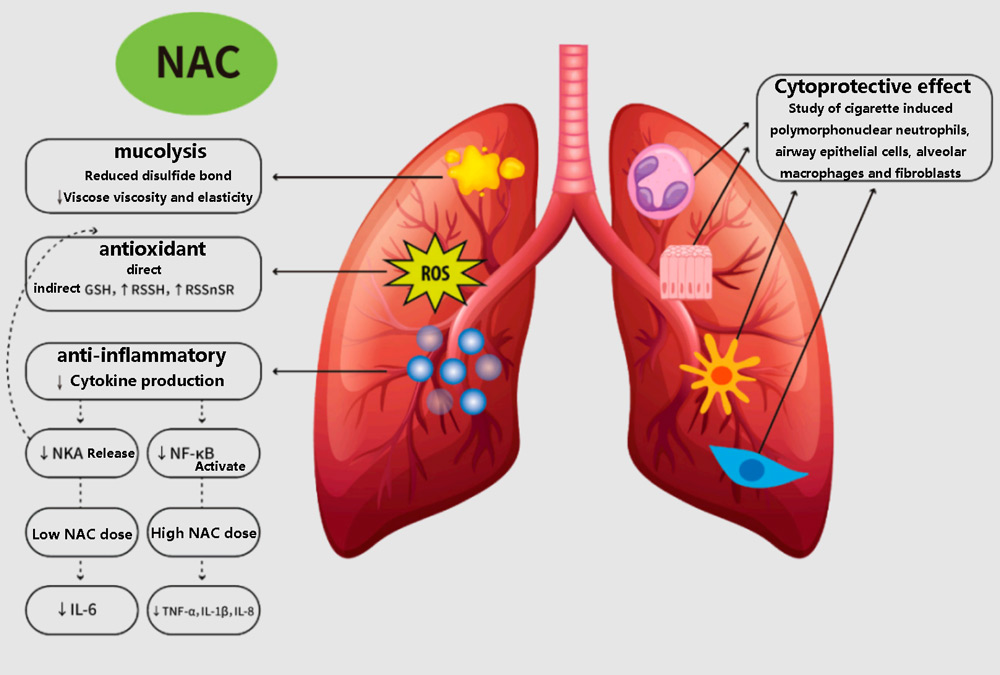
A 2016 meta-analysis of NAC literature concluded that the compound has been shown to significantly improve COPD symptoms, which is not surprising.
A similar study in 2000 found that NAC also improves symptoms of bronchitis, in part because its up-regulation of GSH can actually dilute mucus produced by the respiratory tract.
Of course, NAC has proven its golden worth over the past few years, with incredible results in fighting more “modern” respiratory diseases.
The progrowth effect of NAC
Most of us have probably heard by now that oxidative stress can also seriously damage fertility – for both people of all genders.
One particularly interesting study that investigated the effect of NAC on this was conducted in men with varicocele.
Varicocele is a disease of varicose veins in the testicles that causes blood to return and a buildup of toxins in the testicular tissue. In some cases, varicocele can negatively affect fertility and testosterone production.
The men in the study first had their varicocele surgically corrected and were then randomly assigned to either the NAC group or a placebo group so the researchers could track the impact of each treatment on their fertility.
By the end of the three-month study period, the men who took 450 mg of NAC daily had significantly better sperm quality than those who took a placebo.
In another study, men with varicocele were randomly divided into four groups: those who took 500 milligrams of NAC daily, those who took 200 micrograms of selenium daily, those who took a placebo, and the final group was those who took both 500 milligrams of NAC and 200 micrograms of selenium.
Although sperm quality in the combined NAC and selenium group improved most significantly by the end of the treatment period, the group taking just 450 mg of NAC also saw significant improvements.
Studies have shown that NAC can inhibit the adhesion of endothelial cells to neutrophils and improve the secretion function of prostate.
NAC activates proteolytic enzymes to promote semen liquefaction.
There is also evidence that NAC can improve fertility by improving symptom severity in women with polycystic ovary syndrome (PCOS).
Antidiabetic effect of NAC
Inflammation and oxidative stress also play an important role in the development of insulin resistance, metabolic syndrome, and type 2 diabetes (T2D).
As we mentioned in the introduction, GSH depletion is a feature of metabolic dysfunction and is persistent in patients with severe T2D.
Although NAC does not seem to improve symptoms in people with existing type 2 diabetes, animal studies suggest that it can help prevent the onset of diabetes by normalizing blood sugar and reducing inflammation.
Cardioprotective effect of NAC
Studies have also shown that NAC increases the body’s production of nitric oxide (NO), a gaseous molecule produced by artery cells that causes vasodilation, or the dilation of blood vessels, which improves blood circulation and leads to a drop in blood pressure and resting heart rate.
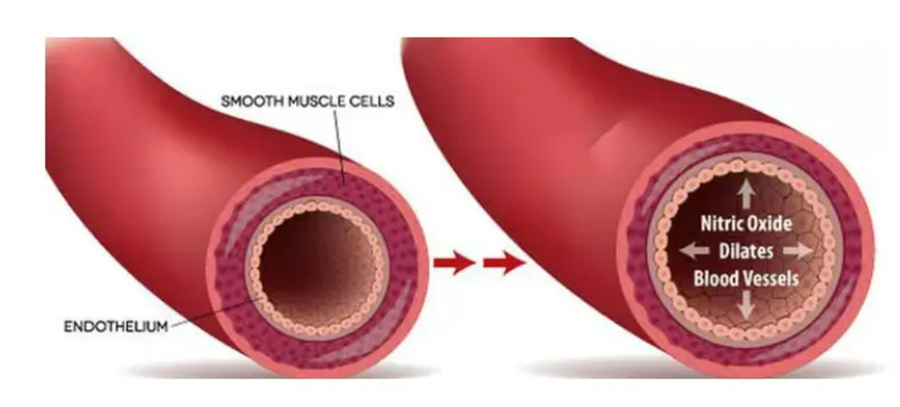
Since reduced NO activity is a feature of cardiovascular disease (CVD), this may help prevent or improve the symptoms of CVD.
NAC boosts immunity
NAC has been shown to help improve the function of the body’s immune system and has been very useful over the past few years.
According to one study, NAC can “fine-tune” the immune response independently of its antioxidant effects.
According to the authors of the study, the GSH produced by NAC supplements not only fights oxidative stress, but also plays an important signaling role for the immune system.
This may be related to the molecular sulfur abundant in NAC regulating immune function through H2S signaling pathway.
Pharmacological effects of acetylcysteine
The antioxidant effect
NAC, as a thiol compound, can react directly with both free radical and non-free radical oxidants, and its reaction rate to oxidants is significantly higher than that of endogenous antioxidants.
Because the molecule contains sulfhydryl group (-SH), it can capture unpaired electrons, remove oxygen free radicals (ROS), and reduce the two bonds in biological macromolecules (-S-S-) through the oxidation of its own -SH, which plays an antagonistic role in oxidation.
Hepatoprotective effect
NAC can dilate blood vessels, improve blood perfusion in the liver, correct tissue hypoxia, ischemia, prevent liver cell necrosis, and protect the liver.
NAC can also improve the mitochondrial autophagy function of hepatocytes induced by free fatty acids in the mitochondrial tricarboxylic acid cycle, and alleviate the symptoms of hepatocyte inflammation.
Promote the synthesis of intracellular glutathione
Glutathione is an important substance in cells. NAC enters the cell, deacetylates and biosynthesizes GSH in the cell, thereby increasing the content of GSH in the cell.
NAC can also convert oxidized glutathione to reduced glutathione, promote its antioxidant capacity, and achieve the purpose of preventing cell damage.
Protective nucleic acid molecule
NAC can protect the body by repairing DNA damage, inhibiting genotoxicity and cell transformation.
It also inhibits the direct mutagenicity of reactive oxygen species (ROS) and DNA breakage caused by peroxide.
immunomodulation
NAC has a strong antioxidant and increase the level of T cell CD4, so it has the effect of improving immunity.
Apoptosis regulation
NAC has the effect of anti-peroxide damage, which increases the content of SOD and glutathione, reduces the oxidative stress reaction, and thus inhibits the level of apoptosis.
Inhibit inflammation
The function of NAC is to capture electrons directly through the active group in the molecule and inhibit the formation of superoxide anion. The indirect reason is that the blocking of intracellular reactive oxygen species (ROS) leads to the activation of stress active enzymes and the overexpression of vulvae inflammation.
NAC improves gut health
Chronic inflammation underlies many intestinal diseases, including intestinal permeability or leaky gut, which is one of three factors that must be present for autoimmune diseases such as Hashimoto’s to occur.
Intestinal leakage involves damage to the lining of the body’s intestines and causes tight connection failure, which allows materials such as bacteria and food particles that are not intended to pass through the intestinal wall to pass through.
Studies have shown that NAC improves intestinal tissue damage by generating signals to tighten connections within the intestinal wall, just as it does when the gut leaks. This, in turn, patched the “leak” of intestinal leakage.
When the intestinal tissue damage heals and the intestinal leak is reversed, many people may even be able to relieve their Hashimoto!
NAC can also promote gut health by helping gut bacteria detoxify and break down biofilms. Biofilms are collections of microorganisms that grow on the surface of living organisms and often house intestinal pathogens, which in turn can cause infections.
Many biofilms are becoming resistant to many clinical antimicrobial treatments and host immune responses, so researchers are looking for new substances to combat these resistant biofilms.
A 2014 study investigated the effectiveness of NAC in preventing biofilm formation as well as destroying existing biofilms.
The study found that in combination with different antibiotics, NAC can significantly penetrate the deepest layers of bacterial biofilms, which are increasingly resistant to classical antimicrobial treatments.
References:
- Richie, John P Jr et al. “Randomized controlled trial of oral glutathione supplementation on body stores of glutathione.” European journal of nutrition vol. 54,2 (2015): 251-63. doi:10.1007/s00394-014-0706-z https://dx.doi.org/10.1007/s00394-014-0706-z
- Exner, R et al. “Therapeutic potential of glutathione.” Wiener klinische Wochenschrift vol. 112,14 (2000): 610-6.
- Chen, Jinghan Jenny, et al. “Altered Central and Blood Glutathione in Alzheimer’s Disease and Mild Cognitive Impairment: A Meta-Analysis.” Alzheimer’s Research & Therapy, vol. 14, no. 1, 5 Feb. 2022, p. 23, pubmed.ncbi.nlm.nih.gov/35123548/, 10.1186/s13195-022-00961-5 https://alzres.biomedcentral.com/articles/10.1186/s13195-022-00961-5
- Mandal, Pravat K et al. “Cognitive Improvement with Glutathione Supplement in Alzheimer’s Disease: A Way Forward.” Journal of Alzheimer’s disease : JAD vol. 68,2 (2019): 531-535. doi:10.3233/JAD-181054
- Lutchmansingh, Fallon K et al. “Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycemia.” PloS one vol. 13,6 e0198626. 7 Jun. 2018, doi:10.1371/journal.pone.0198626 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5991679/
- Sekhar, Rajagopal V et al. “Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine.” Diabetes care vol. 34,1 (2011): 162-7. doi:10.2337/dc10-1006 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3005481/
- Witschi, A et al. “The systemic availability of oral glutathione.” European journal of clinical pharmacology vol. 43,6 (1992): 667-9. doi:10.1007/BF02284971 https://dx.doi.org/10.1007/BF02284971
- Schmitt, Bernard et al. “Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: A comparative crossover study.” Redox biology vol. 6 (2015): 198-205. doi:10.1016/j.redox.2015.07.012 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4536296/
- Pizzino, Gabriele et al. “Oxidative Stress: Harms and Benefits for Human Health.” Oxidative medicine and cellular longevity vol. 2017 (2017): 8416763. doi:10.1155/2017/8416763 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5551541/
- Lugrin, Jérôme et al. “The role of oxidative stress during inflammatory processes.” Biological chemistry vol. 395,2 (2014): 203-30. doi:10.1515/hsz-2013-0241 https://www.degruyter.com/document/doi/10.1515/hsz-2013-0241
- Khansari, Nemat et al. “Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer.” Recent patents on inflammation & allergy drug discovery vol. 3,1 (2009): 73-80. doi:10.2174/187221309787158371 https://www.eurekaselect.com/930895/article
- Mokhtari, Vida et al. “A Review on Various Uses of N-Acetyl Cysteine.” Cell journal vol. 19,1 (2017): 11-17. doi:10.22074/cellj.2016.4872; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5241507/
- Aydin, Seval, et al. “N-Acetylcysteine Reduced the Effect of Ethanol on Antioxidant System in Rat Plasma and Brain Tissue.” The Tohoku Journal of Experimental Medicine, vol. 198, no. 2, 2002, pp. 71–77, 10.1620/tjem.198.71; https://pubmed.ncbi.nlm.nih.gov/12512991/
- Ozaras, Resat, et al. “N-Acetylcysteine Attenuates Alcohol-Induced Oxidative Stress in the Rat.” World Journal of Gastroenterology, vol. 9, no. 1, 2003, p. 125, 10.3748/wjg.v9.i1.125; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4728225/
- Ozaras, Resat et al. “N-acetylcysteine attenuates alcohol-induced oxidative stress in the rat.” World journal of gastroenterology vol. 9,1 (2003): 125-8. doi:10.3748/wjg.v9.i1.125 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4728225/
- “Elevated Liver Enzymes.” Mayo Clinic, 2018, www.mayoclinic.org/symptoms/elevated-liver-enzymes/basics/definition/sym-20050830
- Quintanilla, María Elena et al. “N-Acetylcysteine and Acetylsalicylic Acid Inhibit Alcohol Consumption by Different Mechanisms: Combined Protection.” Frontiers in behavioral neuroscience vol. 14 122. 31 Jul. 2020, doi:10.3389/fnbeh.2020.00122 https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/32848653/
- Morley, Kirsten C et al. “N-acetyl cysteine in the treatment of alcohol use disorder in patients with liver disease: Rationale for further research.” Expert opinion on investigational drugs vol. 27,8 (2018): 667-675. doi:10.1080/13543784.2018.1501471 https://www.tandfonline.com/doi/full/10.1080/13543784.2018.1501471
- Nguyen-Khac, Eric, et al. “Glucocorticoids PlusN-Acetylcysteine in Severe Alcoholic Hepatitis.” New England Journal of Medicine, vol. 365, no. 19, 10 Nov. 2011, pp. 1781–1789, 10.1056/nejmoa1101214. https://www.nejm.org/doi/full/10.1056/nejmoa1101214
- El-Serafi, Ibrahim et al. “The effect of N-acetyl-l-cysteine (NAC) on liver toxicity and clinical outcome after hematopoietic stem cell transplantation.” Scientific reports vol. 8,1 8293. 29 May. 2018, doi:10.1038/s41598-018-26033-z https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/29844459/
- Ronis, Martin J J et al. “Effects of N-acetylcysteine on ethanol-induced hepatotoxicity in rats fed via total enteral nutrition.” Free radical biology & medicine vol. 39,5 (2005): 619-30. doi:10.1016/j.freeradbiomed.2005.04.011 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2956427/#!po=31.2500
- Ehrig, T et al. “Alcohol and aldehyde dehydrogenase.” Alcohol and alcoholism (Oxford, Oxfordshire) vol. 25,2-3 (1990): 105-16. doi:10.1093/oxfordjournals.alcalc.a044985 https://academic.oup.com/alcalc/article-lookup/doi/10.1093/oxfordjournals.alcalc.a044985
- Wang, Jiali et al. “Inhibition of aldehyde dehydrogenase 2 by oxidative stress is associated with cardiac dysfunction in diabetic rats.” Molecular medicine (Cambridge, Mass.) vol. 17,3-4 (2011): 172-9. doi:10.2119/molmed.2010.00114 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3060979/
- Dean, Olivia et al. “N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action.” Journal of psychiatry & neuroscience : JPN vol. 36,2 (2011): 78-86. doi:10.1503/jpn.100057 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3044191/
- Ambrogini, Patrizia et al. “Excitotoxicity, neuroinflammation and oxidant stress as molecular bases of epileptogenesis and epilepsy-derived neurodegeneration: The role of vitamin E.” Biochimica et biophysica acta. Molecular basis of disease vol. 1865,6 (2019): 1098-1112. doi:10.1016/j.bbadis.2019.01.026 https://linkinghub.elsevier.com/retrieve/pii/S0925-4439(19)30032-8
- Olloquequi, Jordi, et al. “Excitotoxicity in the Pathogenesis of Neurological and Psychiatric Disorders: Therapeutic Implications.” Journal of Psychopharmacology, vol. 32, no. 3, 15 Feb. 2018, pp. 265–275, 10.1177/0269881118754680. https://journals.sagepub.com/doi/10.1177/0269881118754680
- Fernandes, Brisa S et al. “N-Acetylcysteine in depressive symptoms and functionality: a systematic review and meta-analysis.” The Journal of clinical psychiatry vol. 77,4 (2016): e457-66. doi:10.4088/JCP.15r09984 https://pubmed.ncbi.nlm.nih.gov/27137430/
- Paydary, K et al. “N-acetylcysteine augmentation therapy for moderate-to-severe obsessive-compulsive disorder: randomized, double-blind, placebo-controlled trial.” Journal of clinical pharmacy and therapeutics vol. 41,2 (2016): 214-9. doi:10.1111/jcpt.12370 https://doi.org/10.1111/jcpt.12370
- Pósfai, B et al. “Synaptic and cellular changes induced by the schizophrenia susceptibility gene G72 are rescued by N-acetylcysteine treatment.” Translational psychiatry vol. 6,5 e807. 10 May. 2016, doi:10.1038/tp.2016.74 https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/27163208/
- Gray, Kevin M et al. “N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study.” The American journal on addictions vol. 19,2 (2010): 187-9. doi:10.1111/j.1521-0391.2009.00027.x https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2826714/
- Berk, Michael et al. “The promise of N-acetylcysteine in neuropsychiatry.” Trends in pharmacological sciences vol. 34,3 (2013): 167-77. doi:10.1016/j.tips.2013.01.001 https://linkinghub.elsevier.com/retrieve/pii/S0165-6147(13)00002-3
- Zinellu, Elisabetta et al. “Glutathione Peroxidase in Stable Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-analysis.” Antioxidants (Basel, Switzerland) vol. 10,11 1745. 30 Oct. 2021, doi:10.3390/antiox10111745 https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/34829616/
- Beeh, K M et al. “Glutathione deficiency of the lower respiratory tract in patients with idiopathic pulmonary fibrosis.” The European respiratory journal vol. 19,6 (2002): 1119-23. doi:10.1183/09031936.02.00262402 https://pubmed.ncbi.nlm.nih.gov/12108866/
- Roum, J H et al. “Systemic deficiency of glutathione in cystic fibrosis.” Journal of applied physiology (Bethesda, Md. : 1985) vol. 75,6 (1993): 2419-24. doi:10.1152/jappl.1993.75.6.2419 https://journals.physiology.org/doi/10.1152/jappl.1993.75.6.2419
- Sanguinetti, Claudio M. “N-acetylcysteine in COPD: why, how, and when?.” Multidisciplinary respiratory medicine vol. 11 8. 3 Feb. 2016, doi:10.1186/s40248-016-0039-2 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4744393/
- Stey, C., et al. “The Effect of Oral N-Acetylcysteine in Chronic Bronchitis: A Quantitative Systematic Review.” European Respiratory Journal, vol. 16, no. 2, 1 Aug. 2000, pp. 253–262 https://erj.ersjournals.com/content/16/2/253.short
- Izquierdo-Alonso, José Luis, et al. “N-Acetylcysteine for Prevention and Treatment of COVID-19: Current State of Evidence and Future Directions.” Journal of Infection and Public Health, vol. 15, no. 12, Dec. 2022, pp. 1477–1483, 10.1016/j.jiph.2022.11.009; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9651994/
- Shi, Zhongcheng, and Carlos A Puyo. “N-Acetylcysteine to Combat COVID-19: An Evidence Review.” Therapeutics and Clinical Risk Management, vol. Volume 16, Nov. 2020, pp. 1047–1055, 10.2147/tcrm.s273700; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7649937/
- Çayan, Selahittin et al. “Effect of Varicocele and Its Treatment on Testosterone in Hypogonadal Men with Varicocele: Review of the Literature.” Balkan medical journal vol. 37,3 (2020): 121-124. doi:10.4274/balkanmedj.galenos.2020.2020.1.85 https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/32070086/
- Kupis, Łukasz et al. “Varicocele as a source of male infertility – current treatment techniques.” Central European journal of urology vol. 68,3 (2015): 365-70. doi:10.5173/ceju.2015.642 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4643713/
- Barekat, Foroogh et al. “A Preliminary Study: N-acetyl-L-cysteine Improves Semen Quality following Varicocelectomy.” International journal of fertility & sterility vol. 10,1 (2016): 120-6. doi:10.22074/ijfs.2016.4777 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4845522/
- Safarinejad, Mohammad Reza, and Shiva Safarinejad. “Efficacy of selenium and/or N-acetyl-cysteine for improving semen parameters in infertile men: a double-blind, placebo controlled, randomized study.” The Journal of urology vol. 181,2 (2009): 741-51. doi:10.1016/j.juro.2008.10.015 https://www.auajournals.org/doi/10.1016/j.juro.2008.10.015
- Thakker, Divyesh et al. “N-acetylcysteine for polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled clinical trials.” Obstetrics and gynecology international vol. 2015 (2015): 817849. doi:10.1155/2015/817849 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4306416/
- Esser, Nathalie et al. “Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes.” Diabetes research and clinical practice vol. 105,2 (2014): 141-50. doi:10.1016/j.diabres.2014.04.006 https://linkinghub.elsevier.com/retrieve/pii/S0168-8227(14)00187-9
- Szkudlinska, Magdalena A et al. “The antioxidant N-Acetylcysteine does not improve glucose tolerance or β-cell function in type 2 diabetes.” Journal of diabetes and its complications vol. 30,4 (2016): 618-22. doi:10.1016/j.jdiacomp.2016.02.003 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4834245/
- Ma, Yongjie et al. “N-acetylcysteine Protects Mice from High Fat Diet-induced Metabolic Disorders.” Pharmaceutical research vol. 33,8 (2016): 2033-42. doi:10.1007/s11095-016-1941-1 https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/27161488/
- Dludla, Phiwayinkosi V et al. “A Systematic Review on the Protective Effect of N-Acetyl Cysteine Against Diabetes-Associated Cardiovascular Complications.” American journal of cardiovascular drugs : drugs, devices, and other interventions vol. 18,4 (2018): 283-298. doi:10.1007/s40256-018-0275-2 https://dx.doi.org/10.1007/s40256-018-0275-2
- Anfossi, G et al. “N-acetyl-L-cysteine exerts direct anti-aggregating effect on human platelets.” European journal of clinical investigation vol. 31,5 (2001): 452-61. doi:10.1046/j.1365-2362.2001.00815.x https://onlinelibrary.wiley.com/resolve/openurl?genre=article&sid=nlm%3Apubmed&issn=0014-2972&date=2001&volume=31&issue=5&spage=452
- Naseem, Khalid M. “The role of nitric oxide in cardiovascular diseases.” Molecular aspects of medicine vol. 26,1-2 (2005): 33-65. doi:10.1016/j.mam.2004.09.003 https://pubmed.ncbi.nlm.nih.gov/15722114/
- Diotallevi, Marina et al. “Glutathione Fine-Tunes the Innate Immune Response toward Antiviral Pathways in a Macrophage Cell Line Independently of Its Antioxidant Properties.” Frontiers in immunology vol. 8 1239. 29 Sep. 2017, doi:10.3389/fimmu.2017.01239 https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/29033950/
- Rodrigues, Camila, and Susan Percival. “Immunomodulatory Effects of Glutathione, Garlic Derivatives, and Hydrogen Sulfide.” Nutrients, vol. 11, no. 2, 30 Jan. 2019, p. 295, 10.3390/nu11020295. Accessed 19 Mar. 2020. https://www.mdpi.com/2072-6643/11/2/295/htm
- Hildebrandt W, Alexander S, Bartsch P, Droge W. Effect of N-acetyl-cysteine on the hypoxic ventilatory response and erythropoietin production: linkage between plasma thiol redox state and O(2) chemosensitivity. Blood. 2002 Mar 1;99(5):1552-5. 86.Iturriaga R, Rey S, D


