The research team realized that biocompatible nanoparticle (NPS) are already widely used in the field of drug delivery and that several clinical drugs have successfully entered the market, and that the versatile nature of nanoparticles offers new possibilities for the development of TPD tools.
Targeted protein degradation (TPD) is a hot research direction in today’s biomedical field. By TPD molecules, disease-related proteins are pulled to the “recycling station” in the cell for degradation and removal, which provides great therapeutic potential for proteins that are difficult to be cured by traditional drugs.
Existing TPD molecules often require complex molecular design and de novo synthesis to adapt to different disease targets and organ specificity.
On October 28, 2024, the team of Professor Meng Zheng and Bing Yang of Henan University and the team of Professor Jinrong Liang of Columbia University published an important research paper in Nature Nanotechnology (IF 38.1). Entitled “Targeted protein degradation via cellular trafficking of nanoparticles”.
The research team reported a new nanobiological effect, finding that antibody, polypeptide-modified nanoparticle spontaneously initiate targeted protein degradation during cell transport, without the need for special cellular endocytosis and degradation pathways to guide structural design.
Therefore, a nanoparticle based universal strategy for targeted protein degradation (TPD-NPS) is proposed, which is expected to not only change the cumbersome development of existing TPD tools, but also provide new knowledge for nanomedicine and expand the application scope of nanomedicine.
In this study, the researchers propose a general strategy of using engineered nanoparticles (NPS) to design and develop effective TPD tools for the degradation of extracellular target proteins (POI).
In Figure 1, TPD-NP can formed by binding a POI binding ligand to a DSPE-PEG connecting arm and anchoring to a PLGA nucleus.
The researchers prepared NTZ-NP by coupling PEG-DSPE with Nimotuzumab (NTZ), which targets the membrane protein EGFR, and assembled it with PLGA nanoparticles. The results showed that NTZ-NP can effectively promote the degradation of the membrane protein EGFR.
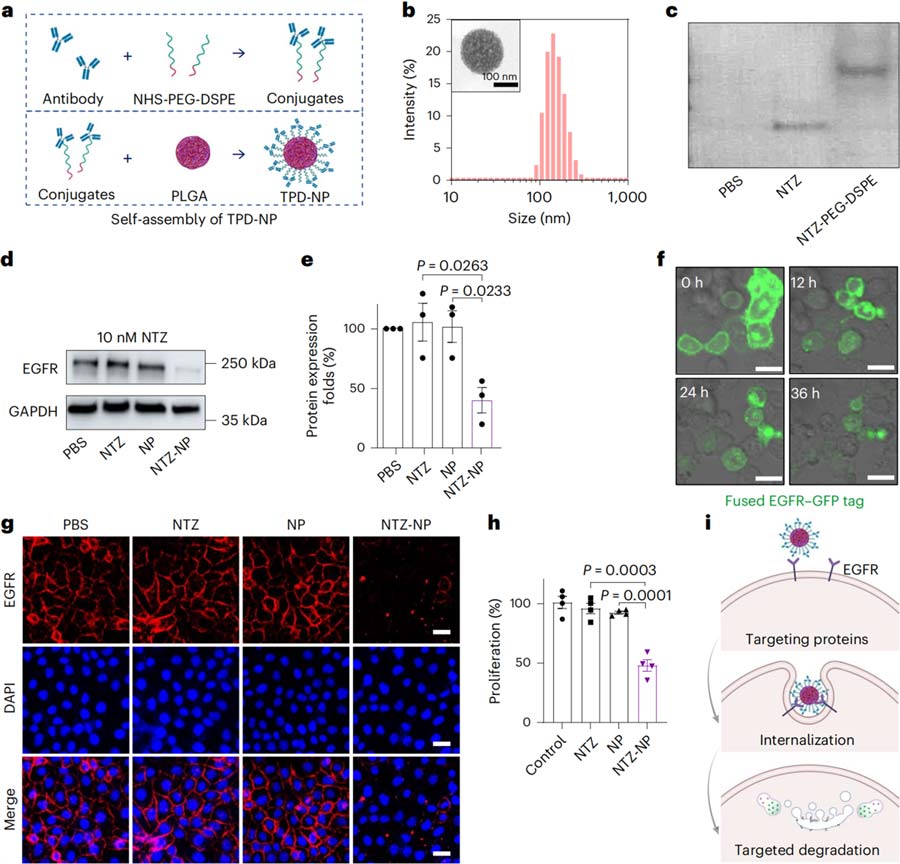
Note: PLGA is a commonly used medical polymer material; NTZ stands for EGFR-targeting antibody nituzumab;
Further research shows that the TPD-NP strategy is universal, applicable to multiple targets and different nanoparticle forms, and therefore has broad application potential (Figure 2).
There are many kinds of target proteins that the TPD-NP system can interfere with, and the targets that have tested include the tumor immune escape target PD-1/PD-L1 and tumor markers such as EGFR and HER2.
The “target head” design of TPD-NP is flexible and changeable, in addition to antibodies, it can also bind target proteins through a variety of ways such as peptides, such as ACE2, a key cell receptor for SARS-CoV-2, influenza virus receptor and tumor surface marker CD13.
TPD-NP also shows great flexibility in the selection of nanoparticles.
Many types of nanocarriers such as liposomes, exosomes, biomimetic nanoparticles and inorganic nanoparticles can used to construct TPD-NP systems.
This diversity allows the TPD-NP system to combined and optimized for different therapeutic needs, easily building a variety of highly effective targeted protein degradation tools.
The research team has successfully developed a TPD-NP system that can degrade multiple targets simultaneously, and designed a TPD-NP system with tissue-specific targeting capabilities.
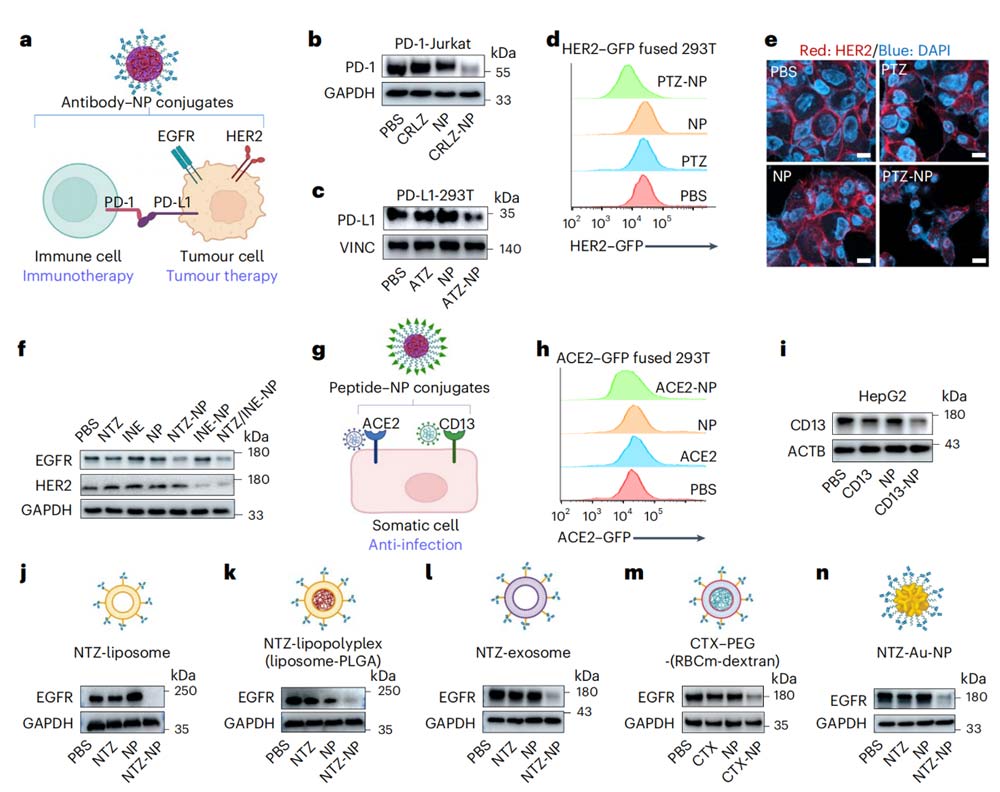
Note:
- CRLZ refers to PD-1 targeting antibody Camrelizumab;
- ATZ stands for PD-L1 targeting antibody Atezolizumab;
- PTZ and INE refer to HER2-targeting antibodies Pertuzumab and Inetetamab;
- ACE2 and CD13 represent their respective binding peptides.
- CTX stands for EGFR-targeting antibody Cetuximab;
- RBCm stands for erythrocyte membrane;
- Dextran is a commonly used medical material.
- Au-NP stands for gold nanoparticles.
A variety of nanoparticle forms used in j-n, including liposomes, liposome polymer complexes, exosomes, cell membranes, gold nanoparticles, etc., all of which can connect antibody and other targeting groups through PEG-lipids.
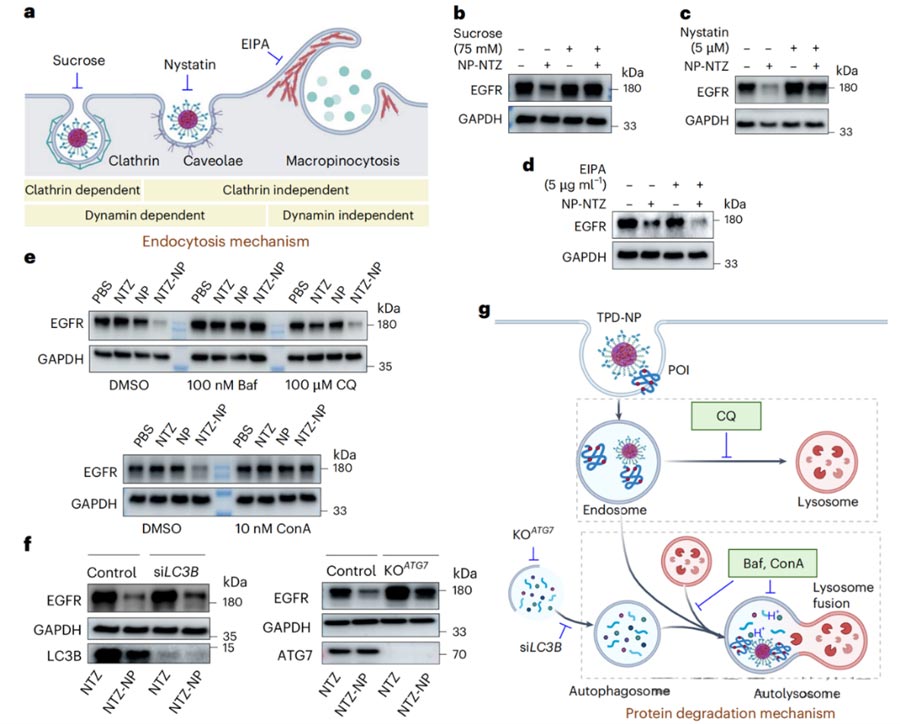
This study also explored in depth the specific mechanism of targeted protein degradation by TPD-NP (Figure 3).
TPD-NP enters the cell mainly through clathrin and fossuloprotein mediated endocytosis pathway, rather than relying on macropinocytosis mechanism.
After entering the cell, TPD-NP degrades the target protein via the autophagolysosomal pathway, rather than the proteasome pathway.
To verify the actual effects of TPD-NP, the research team conducted experiments in mouse models of human triple-negative breast cancer and human glioma (Figure 4).
The results showed that the modified TPD-NP could successfully degrade the key protein of tumor in mice, effectively inhibit tumor growth and significantly prolong the survival of mice.
These results show that TPD-NP has great potential to enable personalized therapy through a “plug and play” approach.
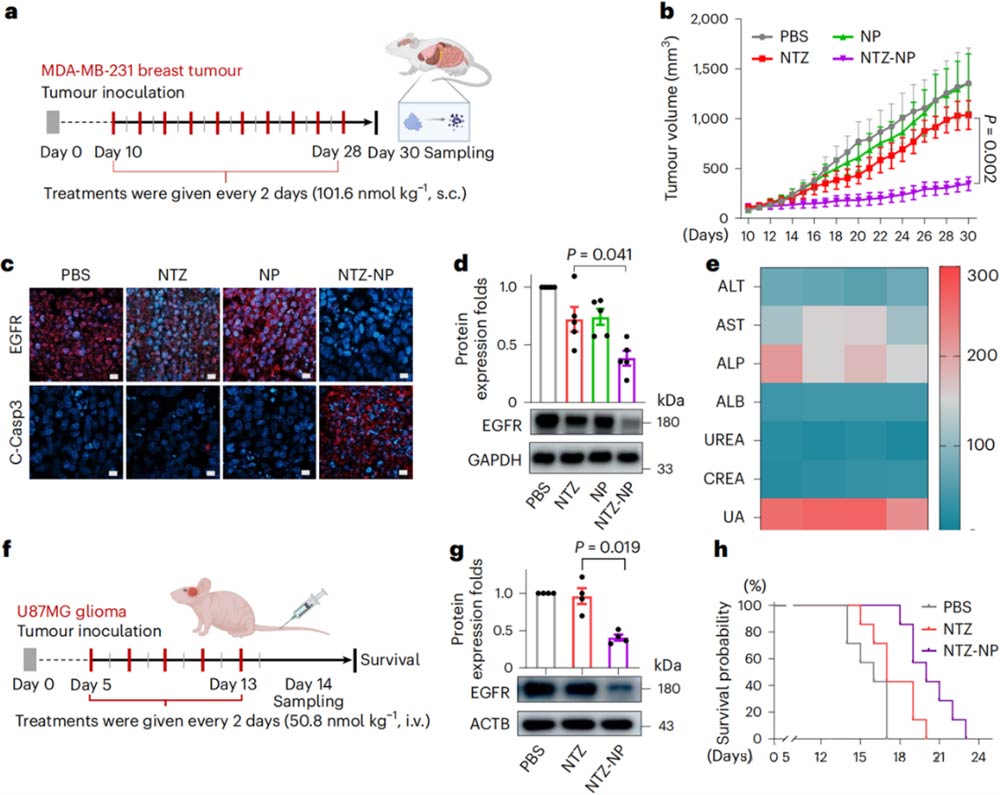
a, b, c, d. Treatment of subcutaneous breast cancer model mice by NTZ-NP; e. Evaluation of the liver and kidney burden of mice by NTTZ-NP; f, g, h. NTZ-NP for the treatment of brain glioma mice
In general, this study reveals a new nanobiology effect of nanoparticles degrading target proteins, which provides a new basic knowledge and direction for the design and development of nanomedicine.
The research also suggests a broader platform for targeted protein degradation, while advancing the development of drug nanodelivery and TPD technologies.
In the future, with the deepening of research and the continuous maturity of technology, TPD-NP expected to play a more important role in the treatment of a variety of diseases.
The original link: https://doi.org/10.1038/s41565-024-01801-3