By activating the NAD+/SIRT3 signaling pathway, β-NMN can reduce inflammation and apoptosis in sepsis related acute kidney injury, protect kidney function, provide a new strategy for treatment, and provide a scientific basis for clinical application.
Sepsis is a systemic inflammatory response caused by infection, often leading to multiple organ dysfunction, of which acute kidney injury (AKI) is a common complication.
β-nicotinamide mononucleotide (β-NMN), as a precursor of NAD+, has shown potential in fighting sepsis.
On November 23, 2024, a study published in the international journal Cell Biochemistry and Biophysics explored how β-NMN alleviates sepsis related acute kidney injury at the molecular level.

β-NMN increases NAD+ levels and reduces inflammation and apoptosis
In the study, β-nicotinamide mononucleotide (β-NMN) was used in a mouse model of sepsis associated acute kidney injury (AKI).
By intraperitoneal injection, β-NMN was given to mice immediately after cecal ligation and perforation surgery (CLP).
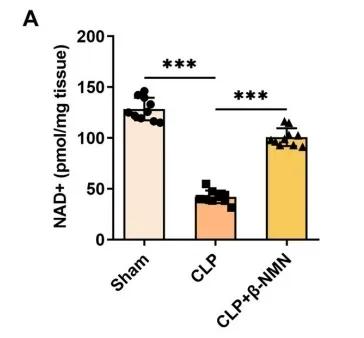
The results showed that treatment with β-NMN significantly increased the NAD+ content in mouse kidney tissues compared with the control group (CLP group).
In terms of tissue structure, the glomeruli and renal tubules of mice in the β-NMN group were clearly and completely structured, similar to those in the Sham group that did not undergo any surgical procedures, and the protection of this structure suggests that β-NMN has a protective effect on the kidneys.
The renal damage caused by caecal ligation and perforation (CLP), such as the expansion of renal tubules, contraction of glomeruli, epithelial cell disturbance, and infiltration of inflammatory cells, significantly improved by β-NMN supplementation.
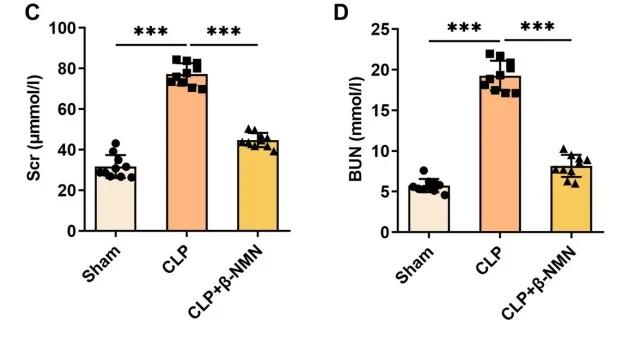
Compared with the control group (CLP group), the β-NMN group also significantly reduced the serum creatinine (Scr) and blood urea nitrogen (BUN) levels, and the reduction of these two indicators indicated that β-NMN had a protective effect on renal function.
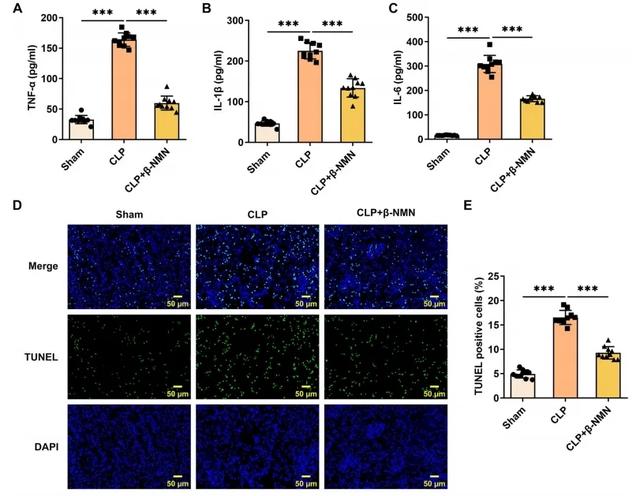
In terms of inflammatory factors, the levels of tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) in the β-NMN group were significantly lower than those in the control group.
These inflammatory factors play a key role in the development of sepsis and acute kidney injury (AKI), and β-NMN mitigated the inflammatory response by reducing their levels.
In addition, the decrease in the number of positive cells associated with self-destruction (apoptosis) and the decreased expression of proteins related to cell death suggest that β-NMN also mitigated renal cell apoptosis induced by cecal ligation and perforation surgery (CLP).
These results suggest that β-NMN effectively alleviates inflammation and apoptosis in mice with sepsis associated acute kidney injury (AKI) by increasing NAD+ levels, thereby protecting kidney function.
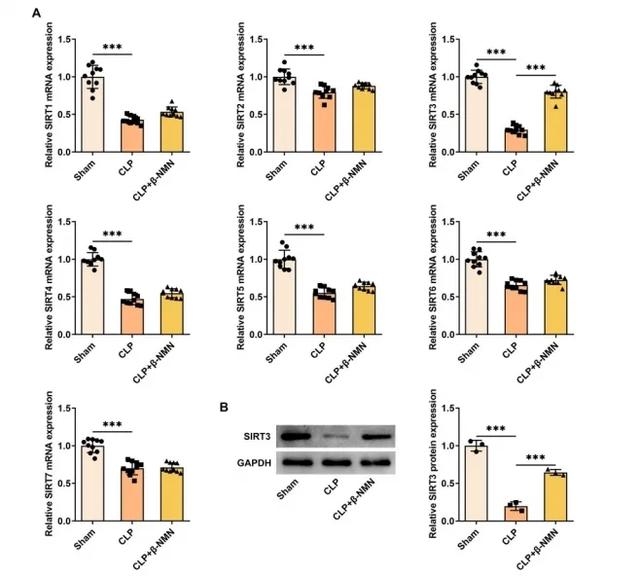
β-NMN up-regulates SIRT3 expression and protects kidney function
In a mouse model of sepsis associated acute kidney injury (AKI), β-NMN significantly upregulated SIRT3 mRNA and protein expression in kidney tissue.
SIRT3 is a NAD+ dependent deacetylase that plays an important role in regulating inflammation, apoptosis and energy metabolism.
By increasing the expression of SIRT3, β-NMN may enhance its inhibitory effect on inflammation and apoptosis, thereby protecting kidney function.
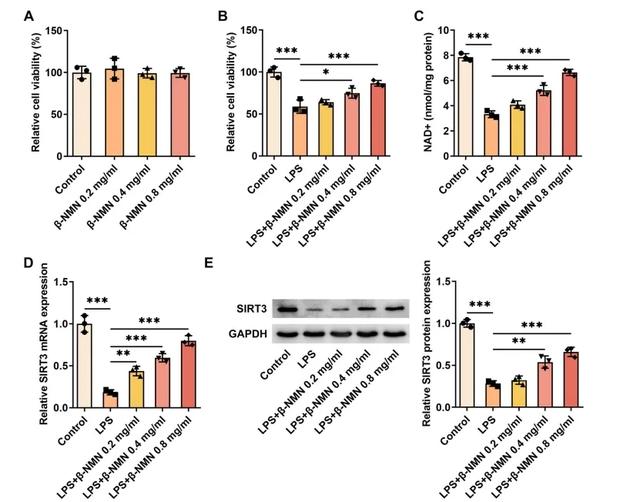
In vitro experiments, β-NMN treatment also increased SIRT3 expression in HK-2 cells stimulated by LPS, a bacterial component capable of mimicking the state of sepsis.
HK-2 cells a human tubular epithelial cell line commonly used in the study of kidney disease.
β-NMN upregulated SIRT3 expression in these cells, suggesting that it may reduce lipopolysaccharids-induced inflammation and cell damage by activating the NAD+/SIRT3 signaling pathway.
Sum up
Overall, β-NMN mitigated kidney inflammation and apoptosis in sepsis associated acute kidney injury (AKI) by activating the NAD+/SIRT3 signaling pathway, providing a new strategy for the treatment of sepsis associated acute kidney injury (AKI).
These findings not only deepen our understanding of the role of NMN at the molecular level, but also provide a scientific basis for future clinical applications.