Recently, Raul Mostoslavsky’s team, from Harvard Medical School in The US, published a paper online in Nature Cell Biology entitled “The glutathione S-transferase Gstt1 drives survival and Survival” dissemination in metastases is to carry out RNA sequencing (RNA-SEQ) and subsequent lost-function shRNA targeting for matched primary and metastatic solid tumors from PDAC and breast cancer (BC) mouse models by using in situ metastasis models.
In order to establish metastases, cancer cells from the primary tumor need to overcome many obstacles – crossing blood vessels, surviving in circulation, and eventually “settling” in a new habitat, a process that requires different molecular programs to give cancer cells the ability to adapt to the metastatic niche and develop significant metastases, although this seems to be an extremely inefficient process. However, metastasis still accounts for 90% of cancer-related deaths.
Determining the adaptive mechanisms of metastatic cancer cells remains an open question, especially for pancreatic cancer (PDAC).
In recent years, there has been a growing focus on identifying early metastatic drivers of primary tumors.
Metastatic spread is considered to be an early event in the progression of various types of cancer, and this is particularly evident in PDAC, where most patients present with extrapancreatic invasive and metastatic disease at initial diagnosis, with a 5-year survival rate of only ~7.1%.
Metastatic lesions have been found to lack additional driver mutations in PDAC compared to primary tumors.
With great biological heterogeneity among primary tumors, disseminated disease,
and significant metastases, combined with synchronous or undetectable metastases,
interventions targeting primary tumor transmission mechanisms are unlikely to be clinically successful.
Elucidating the key regulatory pathways that distinguish metastatic tumors from primary tumors is a pressing issue in the field.
The discovery that glutathione S-transferase Gstt1 is necessary for tumor spread and metastasis,
but is optional for the growth of primary tumors, demonstrates the heterogenous expression pattern of Gstt1 in metastatic tumors,
reveals the key molecular mechanism of Gstt1 to maintain the metastatic growth of cancer cells, and thus identifies Gstt1 as a key driver of metastasis.
It is important to emphasize the effect of tumor cell heterogeneity on the metastasis microenvironment.
In an attempt to identify genes required for the late stages of the metastatic cascade (i.e., metastatic growth and maintenance) based on transcriptional differences between primary tumors and large metastases,
the researchers first constructed mouse PDAC and BC models,
obtained matched primary and metastatic tumor tissues, and treated them with RNA-seq.
The analysis revealed a common differential up-regulation gene signature in liver and lung metastases compared to paired primary tumors.
To functionally test whether this upregulated “metastasis signature” is necessary for the growth and survival of established metastatic cells, we developed a targeted 96-well shRNA screening method under anchored independent growth conditions and successfully identified 17 genes associated with anchored independent survival of metastatic tumor cells.
Among them, the researchers found that Gstt1, which encodes the glutathione S-transferase (GST) θ1 enzyme (which is a member of the protein superfamily that catalyzes the binding of reduced glutathione (GSH) to various electrophilic and hydrophobic compounds),
is essential for the formation of tumor spheroids and is required for the anchoring independent growth of metastatic cells.
However, it is dispensable under two-dimensional (2D) adhesion conditions.
Given that the current functional studies that directly link Gstt1 to tumorgenesis, progression,
and metastatic disease are still inconclusive, it has become the gene that researchers in this paper pay the most attention to.
In this paper, the researchers first confirmed that Gstt1 is a driver of tumor metastasis by using two mouse tumor models,
which is a necessary and sufficient condition to drive metastasis, and its GST activity is necessary for metastasis formation.
The experimental results confirmed that Gstt1 was expressed in metastatic CK19+ tumor cell subsets, but was generally absent in primary tumors.
At the same time, experimental results show that Gstt1 is not essential for primary tumor growth,
but plays a key role in mediating tumor cell spread, which is necessary not only for metastasis formation,
but also for the maintenance of metastatic tumors that have already formed.
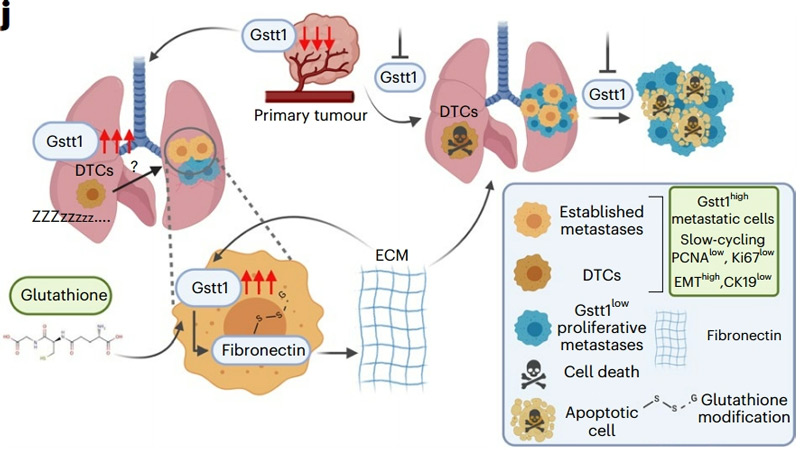
Disseminated tumor cells (DTCS) are single cells or clusters of small cells that escape the primary tumor and reach distant organs.
To assess whether Gstt1 important for tumor spread, we evaluated Gstt1 expression in DTC in liver and lung tissue from a genetically engineered mouse model of PDAC (GEMM, with primary tumors but no significant large metastases),
showing that most individual DTC and small clusters express CK19 and Gstt1 simultaneously.
More importantly, GSTT1-positive DTC was also present in liver tissue from patients with primary PDA who underwent a rapid autopsy without significant metastasis.
Further experimental results confirmed that Gstt1 is necessary for cancer cells to escape from the primary tumor,
which promotes the spread and survival of DTC in the metastatic microenvironment.
At the same time, the researchers found that Gstt1 highly expressed (Gstt1high) cells represented a slow-cycling metastatic subpopulation.
Further analysis of the functional and molecular characteristics of the Gstt1high metastatic subpopulation revealed that Gstt1high cells are a highly metastatic,
slow-cycling subpopulation with enhanced EMT features,
and retain potential disseminative cell features and enhanced metastatic initiation potential in established metastases.
In order to determine the specific function of GSTT1 in metastatic tumors, the researchers then explored its potential interactions and protein substrates, and found that fibronectin plays an important role in Gstt1high metastatic cells as a substrate for Gstt1 in metastatic cells, and Gstt1 can directly modify fibronectin with glutathione to promote metastasis.
Further experimental results revealed a mechanism whereby, in the presence of available glutathione,
Gstt1 expression enhanced in metastatic cells, binding to and glutathione modification of fibritin,
thereby promoting its secretion and deposition into the metastatic microenvironment,
and promoting the formation of extracellular matrix (ECM) that supports the survival of metastatic cells.
It is also this modification of fibronectin by Gstt1, rather than protection against oxidative stress,
that identifies Gstt1 as the key regulator of metastatic disease.
In summary, using a mouse spontaneous metastasis model combined with a newly developed targeted loss-of function screening method,
this study found and confirmed that Gstt1 preferentially upregulated in mouse and human metastases and acts as a specific driver of metastasis without affecting the growth of the primary lesion.
The highly expressed cell subsets of Gstt1 are necessary for the maintenance of established metastases,
thus providing an in-depth understanding of metastatic heterogeneity and a strong theoretical basis for targeting Gstt1 for the treatment of metastatic diseases.
reference
- 1. Lambert, A. W., Pattabiraman, D. R. & Weinberg, R. A. Emerging biological principles of metastasis. Cell 168, 670–691 (2017).
- 2. Rhim, A. D. et al. EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361 (2012).
- 3. Makohon-Moore, A. P. et al. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat. Genet. 49, 358–366 (2017).
- 4. Giancotti, F. G. Mechanisms governing metastatic dormancy and reactivation. Cell 155, 750–764 (2013).




