Efficient recovery of heavy metals and selenium from wastewater using granular sludge: the key role of glutathione (GSH)
Research background
Heavy metals, including copper (Cu), cadmium (Cd), lead (Pb) and zinc (Zn), are highly toxic and persistent pollutants that have significant environmental and health impacts.
And these elements are difficult to get out of the body, they tend to accumulate in various organs. In some industrial effluents, heavy metal cations coexist with anionic contaminants of selenium (Se), including selenites (SeO32-, Se(IV)) and selenates (SeO42-, Se(VI)), complicating the treatment process.
Selenium is an essential trace element for life. Its deficiency is associated with a number of diseases, such as Keshan disease, Keshanbe disease and kwashiorkor. However, excessive intake of selenium can be toxic and can lead to a systemic problem.
Typical examples of heavy metal and selenium co-pollution include wastewater from copper hydrometallurgy and flue gas desulphurization (FGD).
Selenium is often associated with sulfide minerals in copper mines, resulting in high concentrations of heavy metals and selenium in wastewater from copper mining and smelting processes. Heavy metals and selenium are also present in coal.
During combustion, these elements concentrate in the flue gas and enter the wastewater stream during the FGD process. Selenium in coal is mainly converted to selenium dioxide (SeO2) in flue gas, and then hydrolyzed to selenite in lime slurry.
This wastewater must be treated to prevent environmental pollution, damage to ecosystems and potential threats to human health through drinking water and the food chain.
Article introduction
Professor Pan Qiangsheng and others from Zhejiang University of Technology published a paper entitled “Efficient recovery of heavy metals and selenium from wastewater using granular sludge: The crucial role of glutathione (GSH) “research paper, this study describes a glutathione (GSH) enhanced granular sludge technology for the removal and recovery of heavy metals and selenium from wastewater.
The removal rates of copper (Cu), cadmium (Cd) and selenium in wastewater can reach 99.4-99.99%, and the recovery rate can reach 73.2-87.9%. Both long-term reactor operation and short-term stimulation experiments showed that the presence of glutathione significantly increased the recovery of Cu, Cd and Se from sludge.
It was identified as metal selenide (MSe), composed of Cu1.08Se(75.4±1.8%) and CdSe(15.4±1.0%).

It is also significantly rich in glutathione-related genes, including gshA, gshB, and gor, as well as glutathione reductase (GorA).
The results show that GSH-mediated in vitro reaction is the main way of MSe synthesis in sludge.
Given the widespread presence of glutathione in a variety of microorganisms, glutathione-mediated MSe synthesis mechanisms are likely to occur in a variety of environments contaminated by heavy metals and selenium.
Main points of this article
Point one: Reactor operation
The stimulating effect of glutathione.
In order to study the effect of glutathione on the formation of MSe in sludge, batch experiments were carried out before reactor operation.
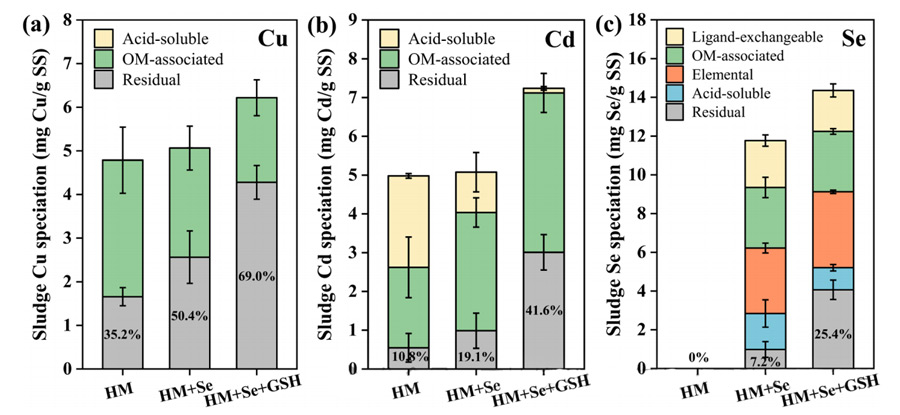
The results showed that the addition of selenite increased the residues of copper (1.7±0.2~2.6±0.6mg/g SS) and Cd(0.5±0.4~0.4~1.0±0.5mg/g SS).
The addition of selenite and glutathione significantly increased the residual fraction of these heavy metals, with Cu reaching 4.3±0.4mg/g SS and Cd reaching 3.0 ± 0.5 mg/g SS.
Notably, the addition of glutathione also significantly increased the residual selenium content, from 1.0 ± 0.4 to 4.1±0.5mg/g SS.
This indicated that glutathione could enhance the formation of residual selenium, which was identified as MSe in subsequent experiments. These findings suggest that glutathione has a similar stimulating effect on the synthesis of cadmium selenide nanoparticles in pure culture of Pseudomonas putida TS44.
Reactor performance.
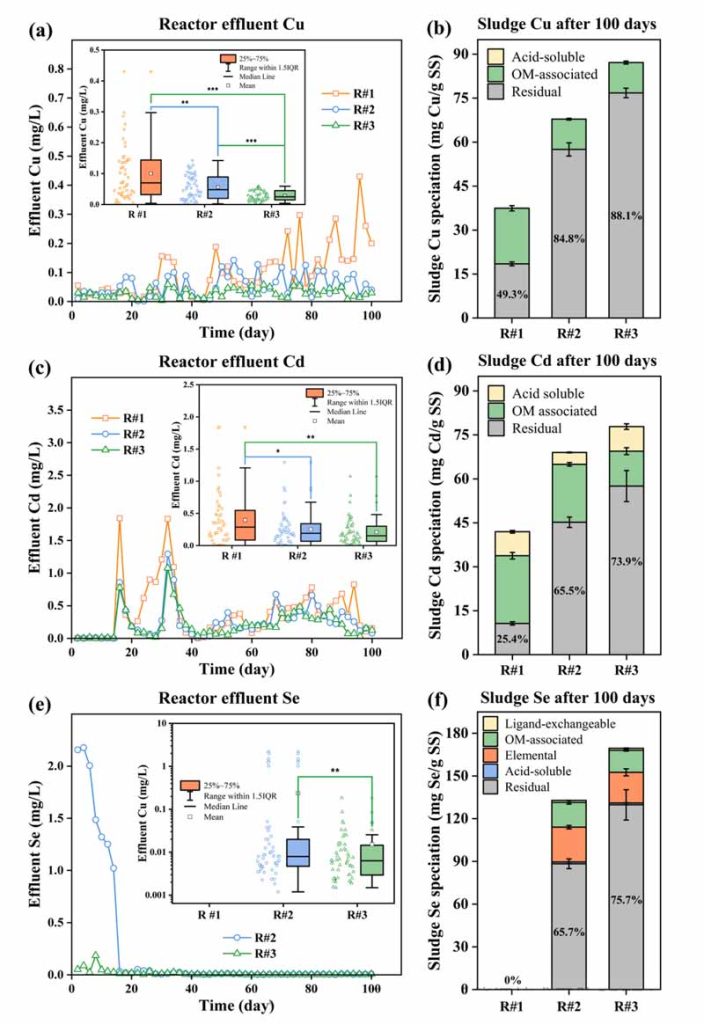
The experimental results show that the effluent Cu of R#3 is significantly lower than that of R#1 and R#2. In the final stage (days 65-100), the Cu concentration in the effluent of R#3 was 0.03±0.02 mg/L, and the removal rate reached 99.9±0.1%. The Cd concentration in the effluent of R#3 is 0.25±0.13 mg/L, and the removal rate is 99.4±0.3%.
The effluent Se concentration of R#3 was significantly lower than that of R#2, especially in the initial stage (days 1-14), when the effluent Se concentration of R#2 was 1.0-2.2 mg/L, while the effluent Se concentration of R#3 rapidly decreased to less than 0.2 mg/L.
This suggests that glutathione can rapidly stimulate the sludge to remove selenite from wastewater.
In the final stage, the effluent Se concentration of R#2 and R#3 was 0.005±0.002 and 0.004±0.002 mg/L, respectively, and the removal rate reached 99.992±0.003% and 99.994±0.003%, respectively.
Formation of elements in sludge.
Compared with R#1, the residual Cu in R#2 sludge increased from 18.5±0.7 to 57.5±2.3 mg/L, and the residual Cd increased from 10.7±0.6 to 45.2±1.8 mg/L.
The residue rates of Cu, Cd and Se in R#3 sludge were the highest, reaching 76.8±1.6, 57.5±5.3 and 129.7±10.6 mg/L, respectively. They accounted for 88.1±0.3%, 73.9±0.2% and 75.7±2.5% of these elements in the sludge, respectively.
These results showed that the presence of selenite increased the residual fraction of heavy metals in the sludge, and the addition of glutathione further increased the residual fraction of heavy metals and selenium.
Point two: Chemical characteristics of sludge
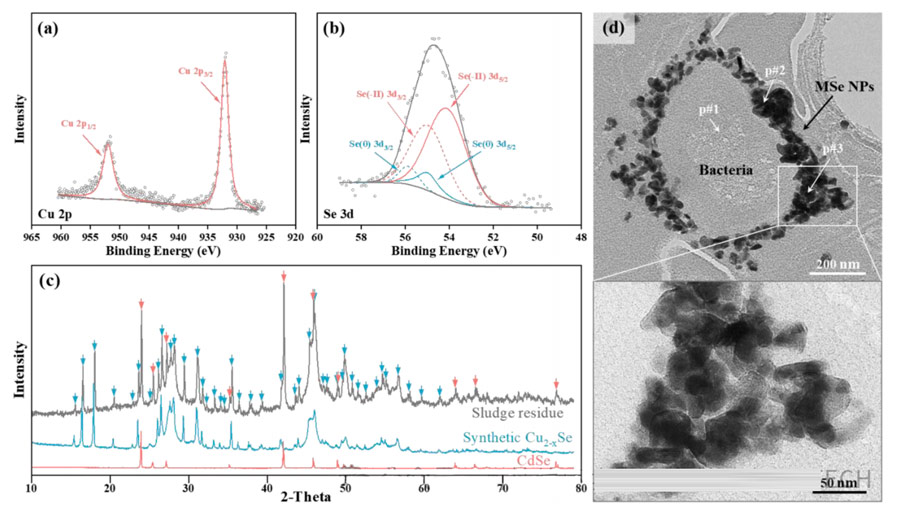
The final sludge of R#3 was sampled, and the Cu 2p spectrum was fitted by a two-state of 2p1/2 and 2p3/2, with a separation of 19.9 eV(spin-orbit splitting) and an area ratio of 1:2.
The characteristic peaks of Cu 2p3/2 and Cd 3d5/2 are 932.09 eV and 404.98 eV respectively, corresponding to the characteristic peaks of CuSe and CdSe, respectively. The Cd bimorph fitted with 3d3/2 and 3d5/2, with a separation of 0.86eV and an area ratio of 2:3.
Two peaks of Se 3d5/2 observed at 54.99 and 54.11 eV, which attributed to Se(0) and Se(-II), respectively. The area ratio of 3d5/2 peaks of Se(0) and Se(-II) is 1:6.83, and the experimental results are consistent with the above.
The mineral composition of sludge Se determined by XRD. There a large number of diffraction peaks in the sludge residue, which can attributed to Cu2-xSe and CdSe(marked with red arrows, PDF#08-0459).
Almost no peaks of any other phase detected, indicating a higher purity of MSe in the residue. Notably, no XRD peaks corresponding to CuS, Cu2S, or CdS detected.
This may be because when selenium species are present, heavy metals tend to form metal selenides rather than sulfides. This may be due to the low solubility of metal selenides in water.
The solubility product constants (Ksp) of CuSe and CdSe were 7.94×10-49 and 6.31×10-36, respectively, and those of CuS and CdS were 6.3×10-36 and 8.0×10-27, respectively.
In summary, the sludge residue mainly composed of MSe, including Cu2-xSe and CdSe, with almost no other mineral impurities.
Point three: the stimulation mechanism of GSH
Stimulation and inhibition experiments.
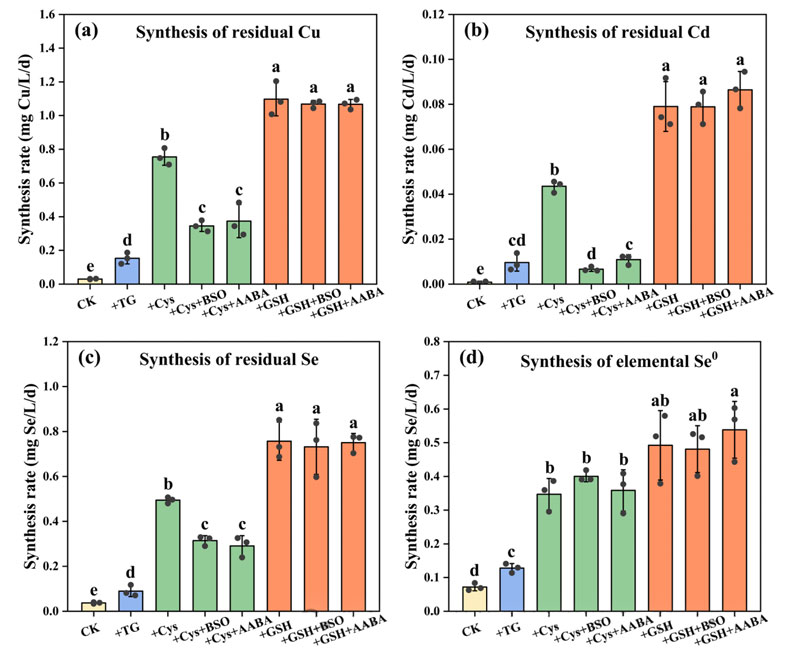
Stimulation experiments showed that the three mercaptans significantly increased the production rates of residual Cu, Cd, Se and Se0 in sludge.
GSH has the strongest stimulating effect, followed by Cys, followed by TG.
Mercaptan can stimulate the synthesis of MSe and Se0 in sludge. In the inhibition experiment, both inhibitors significantly reduced the stimulating effect of Cys on MSe synthesis, but did not reduce the stimulating effect of GSH.
This suggests that GSH is a direct stimulus of MSe synthesis, while Cys may indirectly stimulate this process by converting to GSH.
These findings further confirm the key role of GSH in the synthesis of MSe in sludge.
Neither of the two inhibitors could reduce the stimulating effect of Cys on Se0 synthesis.
This may be because Cys can directly promote the synthesis of Se0 without prior conversion to GSH.
Enzyme reaction in vitro.
The experimental group produced black MSe and red Se0, providing GSH, GorA, and NADPH, respectively. The reaction product dark black-red, indicating that black MSe the main product and red Se0 was less.
In the control group lacking GSH, GorA, or NADPH, there was no synthetic black MSe and red Se0. This indicates that GSH, GorA and NADPH all play key roles in the synthesis of MSe and Se0 in the system.
The forms of heavy metals analyzed. In the experimental group, Cu converted to 79.1±0.9% and Cd to 38.2±3.5%, further proving that Cu more easily synthesized into selenides than Cd.
Key 4: Functional genes and differential expression
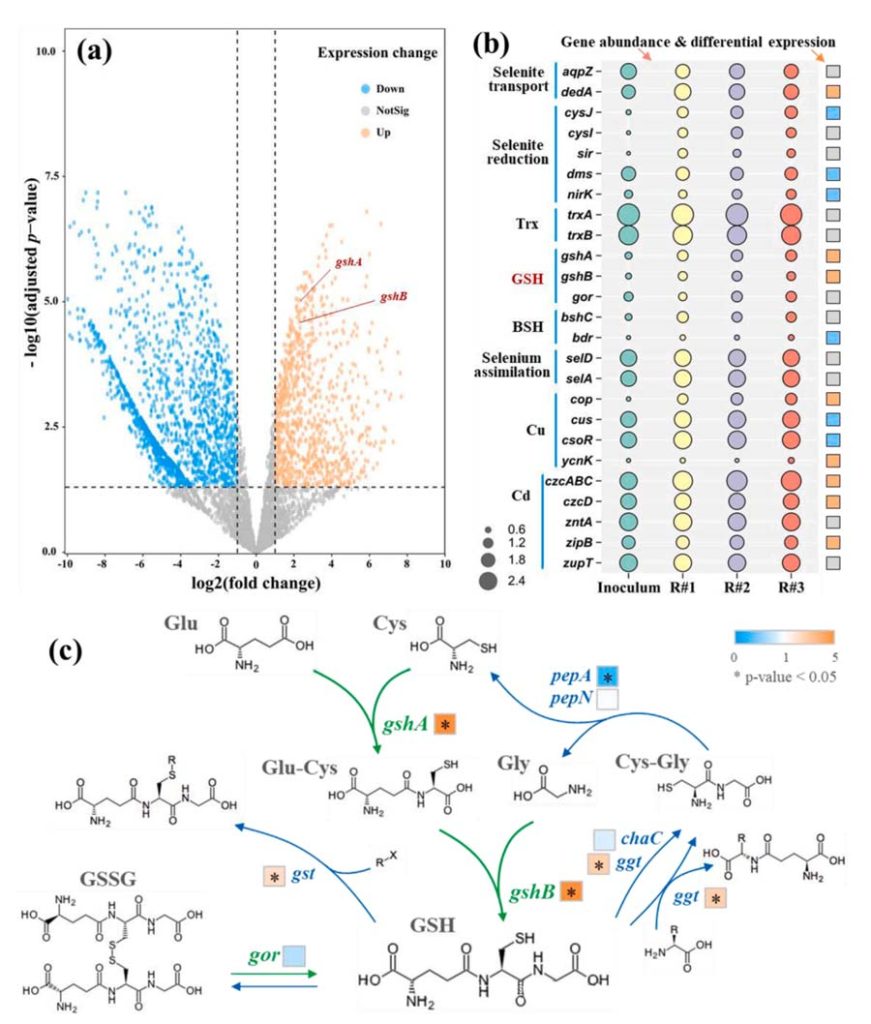
The results showed that 932 genes significantly up-regulated, 1638 genes significantly down-regulated, and 2483 genes had no significant changes.
The gshA and GshB genes encoding the enzyme gshA and glutathione synthase (GshB) involved in glutathione synthesis significantly upregulated.
The microbial pathways of glutathione synthesis, recycling, and degradation shown in Figure 6c.
Initially, GshA catalyzed the synthesis of gamma-glutamylcysteine (Glu-CYS) from L-glutamic acid (Glu) and L-cysteine (Cys).
Subsequently, GshB catalyzes the synthesis of glutathione from Glu-Cys and L-glycine (Gly).
The key genes gshA and gshB significantly up-regulated in the experimental group.
Genes associated with GSH degradation, such as gst and ggt, also significantly upregulated, possibly as a response to elevated GSH levels. There was no significant difference between gor and chaC gene expression.
GorA is encoded by the gene gor, which is responsible for reducing GSSG to glutathione.
The above metagenomic and macrotranscriptomic data further confirm the key role of GSH in Se metabolism in sludge.
conclusion
The addition of glutathione significantly increased the removal rates of heavy metals and selenite from wastewater, Cu was 99.9±0.1%, Cd was 99.4±0.3%, Se was 99.99±0.00%.
In the final R#3 sludge, GSH increased the residual fraction of Cu to 88.1±0.3%, Cd to 73.9±0.2%, and Se to 75.7±2.5%. Residual Cu, Cd, and Se are mainly in the MSe form of Cu2-xSe(Cu1.08Se, 75.4±1.8%) and CdSe(15.4±1.0%).
Stimulation and inhibition experiments have shown that GSH plays a key role in the formation of MSe. Metagenomics and macrotranscriptomic analysis as well as in vitro enzymatic analysis further demonstrated that Delftia is the most active selenite reducing agent in sludge, and glutathione mediated in vitro reaction is the main pathway of MSe synthesis.
Considering that GSH is the most abundant mercaptan among various microorganisms, this mechanism may be widespread in different environments where heavy metals and selenium coexist.
The element recovery rate of the biosynthesized MSe extracted from the final sludge reached 73.2-87.9%. Glutathione enhanced granular sludge technology has shown remarkable performance in the treatment and recovery of copper, cadmium and selenium in wastewater.
reference
Efficient recovery of heavy metals and selenium from wastewater using granular sludge: The crucial role of glutathione (GSH) https://doi.org/10.1016/j.watres.2024.122826