NAD+ is widely used in the field of longevity medicine, doctors in the promotion of the use of NAD+ infusion treatment, but also found a series of problems caused by infusion, and these problems brought clinical manifestations, so that everyone has concerns about the safety of drugs.
At present, the NAD+ products that can exposed include oral NAD+ precursor substances, such as NMN, and NAD+ drugs provided by Cono Pharmaceutical.
The use method recommended by the drug instructions is continuous intramuscular injection for 14 days.
However, the vast majority of institutions use NAD+ intravenous fluid therapy.
The following is a report on a study presented at the A4M annual meeting.
MedRxiv preprint doi: https://doi.org/10.1101/2024.06.06.24308565; posted on June 10, 2024.
About NAD+
Nicotinamide adenine dinucleotide (NAD+) is an essential coenzyme in all living cells and is involved in hundreds of REDOX reactions.
As a reaction substrate, NAD+ plays an important role in metabolic pathways responsible for meeting the energy needs of organisms, including controlling DNA repair, gene expression, and calcium signaling.
NAD+ levels become one of the characteristics of biological aging and can used to assess age-related diseases and physiological changes such as neurodegeneration, hypertension and chronic inflammation.
With increasing age, the concentration of NAD+ in cells and tissues gradually decreases, which is partly due to the accelerated degradation of NAD+ mediated by CD38, resulting in mitochondrial dysfunction.
NAD+ homeostasis also disrupted under conditions of metabolic stress, including heart failure, diabetes, central and peripheral neurodegeneration, mitochondrial disease, alcoholic liver disease, postpartum, and coronavirus infection.
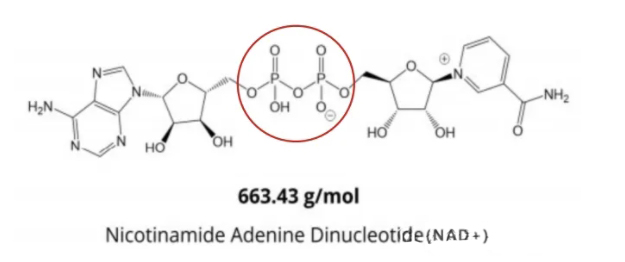
Increasing the level of NAD+ has shown to useful for prevention and treatment, which can save the physiological function defects caused by the absence of NAD+.
Although NAD+ is commercialized as a dietary supplement and as an intravenous drug product, as a pyridine nucleotide, NAD+ itself cannot be absorbed directly through the gut or fully absorbed after extracellular administration.
Most of NAD+ hydrolyzed to nicotinamide mononucleotides (NMN) in the extracellular environment, followed by further cleavage of CD73 to form nicotinamide riboside (NR), some of which may further degraded to nicotinamide and niacin.
NR can rapidly absorbed by cells via equilibrium nucleotides.
Thus, providing exogenous NR, rather than NAD+ itself, appears to enhance intracellular NAD+ concentrations more effectively.
In fact, oral NR may be safer and more effective.
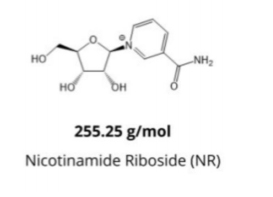
About NAD+ intravenous infusion
Given the above description, oral NAD+ supplementation has limitations.
In recent years, intravenous NAD+ (NAD+IV) has grown in popularity and is available in thousands of boutique medical clinics worldwide.
NAD+ infusion therapy first described in the clinical literature in 1961 to treat a variety of addictive problems and now widely used to promote overall health and longevity.
Effects of NAD+IV include, but not limited to: reducing depression and anxiety, treating drug and alcohol addiction, relieving hangovers, fatigue, neurological disorders, poor athletic performance, and facilitating rapid recovery from the after-effects of COVID-19 and SARS-CoV-2 infections (PASC, “Long coronavirus”).
Intravenous therapy delivers NAD+ directly into the bloodstream, providing 100% bioavailability.

Increasing extracellular NAD+ (eNAD+) may also cause adverse reactions.
Under normal physiological conditions, NAD+ in extracellular fluid has reported to circulate at concentrations of 0.1 to 0.5μM.
eNAD+ exceeding the homeostasis control range may represent a pathophysiological trigger that leads to pro-inflammatory signaling and toxic effects in T cells in clinical models, including apoptosis, and may suppress immune responses.
These findings are particularly noteworthy for NAD+IV, which requires caution due to its ability to enhance NAD+ to superphysiological, potentially pathophysiological levels.
Adverse reactions reported by clinicians include: nausea, diarrhea, muscle cramps, chest pain and lightheadedness.
In light of these concerns, clinical studies have considered other alternative strategies for enhancing NAD+, including alternative products via the intravenous route of administration.
Nicotinamide riboside (NR) has recognized as an endogenous vitamin B3 and NAD precursor, and has become the focus of clinical research.
The patented form of NR chloride, Niagen®, has evaluated for safety by several regulatory agencies, including the U.S.
Food and Drug Administration (FDA), and as a result has received a new dietary ingredient certification.
Oral Niagen® supplements have consistently shown high safety and efficacy, with doses up to 3,000 mg/ day increasing cellular NAD+ levels.
Intravenous administration bypasses gastrointestinal digestive enzyme and microbial-mediated degradation and liver first-pass effects to have a more powerful effect on systemic NAD+ levels.
Multiple sources of evidence support the superior safety profile of intravenous NR (” NR IV “) over that of NAD+IV.
About NAD+IV clinical studies
The objective of this clinical study was to compare the efficacy of a single intravenous injection with NAD+, NR or saline control administration and oral NR supplement in a randomized, double-blind controlled trial.
The objective of the study was to observe changes in whole blood NAD+ levels during and after administration, as well as tolerable infusion rates.
The secondary objective was to compare the clinical safety of NAD+IV and NR IV supplementation.
A total of 37 healthy participants, with an average age of 41, were predominantly white (89%) and 41% female.
The intravenous infusion rate starts at 20 drops per minute to ensure safety and comfort.
NAD+ level test collected fingertip blood.
The test results shown in the figure below:
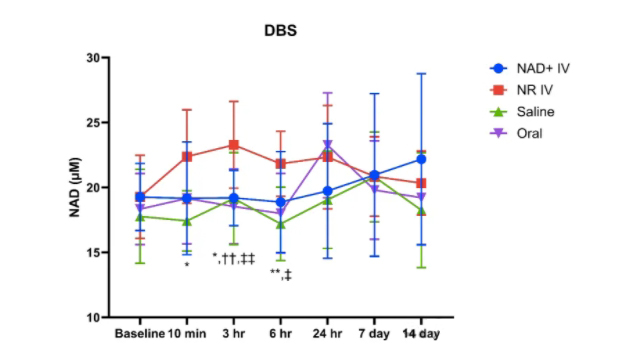
Although the definition of “normal” NAD+ levels in blood and tissues has not determined by scientific means, its generally accepted that maintaining an adequate intracellular pool of NAD+ necessary for optimal health.
Although NAD+IV therapy is popular, safety and efficacy concerns needed, especially since increased eNAD+ may recognized by the immune system as a pathophysiological signal that triggers an inflammatory response.
NAD+IV requires slow administration, and side effects include pain, discomfort, and gastrointestinal disturbance.
A 2019 study found that a total of 750mg of NAD+ administered at an intravenous infusion rate of 2mg/min over 6 hours with no adverse events.
In this study, individual differences found to great, and adverse reactions during NAD+IV included:
Nausea, headache, diarrhea, muscle tension, tingling, mild nausea, and cold.
The adverse reactions disappeared immediately after infusion.
Elevated white blood cells and neutrophils can seen 3 hours after NAD+IV, which may attributed to inflammatory responses, including immunological responses.
Although studies have found that intravenous administration of nicotinamide mononucleotide (NMN, 300 mg dissolved in 100 mL normal saline at an infusion rate of 5 mL/min) was safe and well tolerated in 10 healthy Japanese adults.
The researchers found that intravenous administration of NMN resulted in significant increases in blood NAD+ levels relative to baseline at multiple time points.
NMN is a compound that requires extracellular phosphorylation and is dephosphorylated to form NR or niacinamide, which is easily absorbed by cells to produce NAD+.
Therefore, NR is a more effective way to improve the intracellular NAD+ pool, and NMN is formed after dephosphorylation through extracellular pathways.
Another point of concern in the NAD+IV study is that whole blood NAD+ levels did not increase significantly within 24 hours after infusion, and NAD+ levels increased on days 7 and 14 after infusion.
The difference that whole blood NAD+ levels of NR IV increased significantly at 3-6 hours after infusion, while oral NR observed to increase at 24 hours.
conclusion
In contrast, NR IV administered at a faster rate than NAD+IV, and elevated levels of NAD+ observed within 24 hours after infusion.
However, NAD+IV requires slow intravenous infusion, and an increase in NAD+ levels observed 7-14 days after infusion.
NR IV is safe for intravenous use with no adverse events.
To determine whether to recommend multiple NAD+IV regimentation in the future, or to replace it with NR IV, we need to determine whether nicotinamide riboside provides the same or greater benefits as NAD+IV with fewer side effects and faster intravenous infusion.