Glutathione properties and functions
Glutathione is a tripeptide compound formed by condensation of glutamic acid, cysteine and glycine through peptide bond. Glutathione is the most important low molecular mercaptan to resist oxidative stress in mammalian cells.
In 1921 was discovered,1930 to determine the chemical structure, the famous American nutrition and health care expert Dr. Al Mindel called glutathione for three times the efficacy of anti-aging amino acids, also known as the antioxidant master of nature, the appearance of colorless transparent slender granular crystals, soluble in water, dilute alcohol, liquid ammonia, dimethylformamide, insoluble in ethanol, ether, acetone. Glutathione Factory
Its aqueous solution is easily oxidized to oxidized glutathione in the air, which is widely present in bread yeast, wheat germ, animal liver, chicken blood, pig blood, tomato, pineapple, cucumber, among which wheat germ and animal liver are the highest, the content is as high as 100-1000mg /100g.
It has the functions of anti-oxidation, scavenging free radicals, detoxification, enhancing immunity, delaying aging, anti-cancer and anti-radiation harm. It also helps white blood cells kill bacteria, prevent the oxidation of vitamins C and E, and prevent stroke and cataract formation.
In addition, glutathione has the effect of binding carcinogens and excreting them through the urine.
The liver is the most important detoxification organ in human body, and its rich glutathione (GSH) plays a protective role in liver synthesis, detoxification and estrogen inactivation.
It is the body’s primary antioxidant to counteract damage from free radicals, which are contributing factors to aging and disease.
When the liver is damaged, such as with various liver diseases, the body will consume a large amount of GSH to help the injured liver to repair itself and detoxify, resulting in a significant reduction in glutathione in the body.
At this time, we need to take some glutathione drugs, which help the injured liver to repair itself.
Therefore, glutathione drugs are suitable for viral hepatitis (such as hepatitis A, hepatitis B, etc.), alcoholic liver disease, drug liver disease, fatty liver disease and other liver diseases, and are liver protection medicines for liver disease patients.
Active tripeptide compound
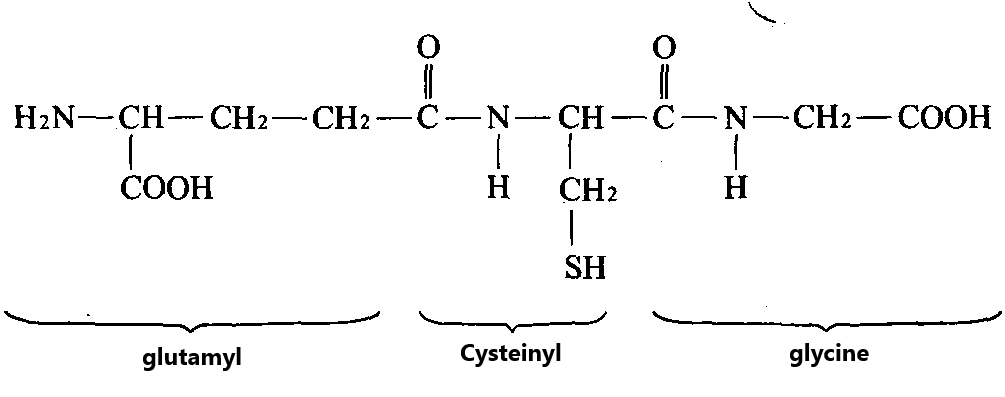
Glutathione (GSH) is a tripeptide compound formed by condensation of glutamic acid, cysteine and glycine through peptide bonds. The chemical name is gamma-l-glutamyl-l-cysteinyl-glycine. Its structural formula is shown in Figure 1.
As can be seen from the structural formula in the figure, GSH is different from other peptides and proteins in that its molecule has a special peptide bond, which is a peptide bond formed by condensation of γ-carboxyl group (-COOH) of glutamic acid and α-amino -(-NH2) of cysteine.
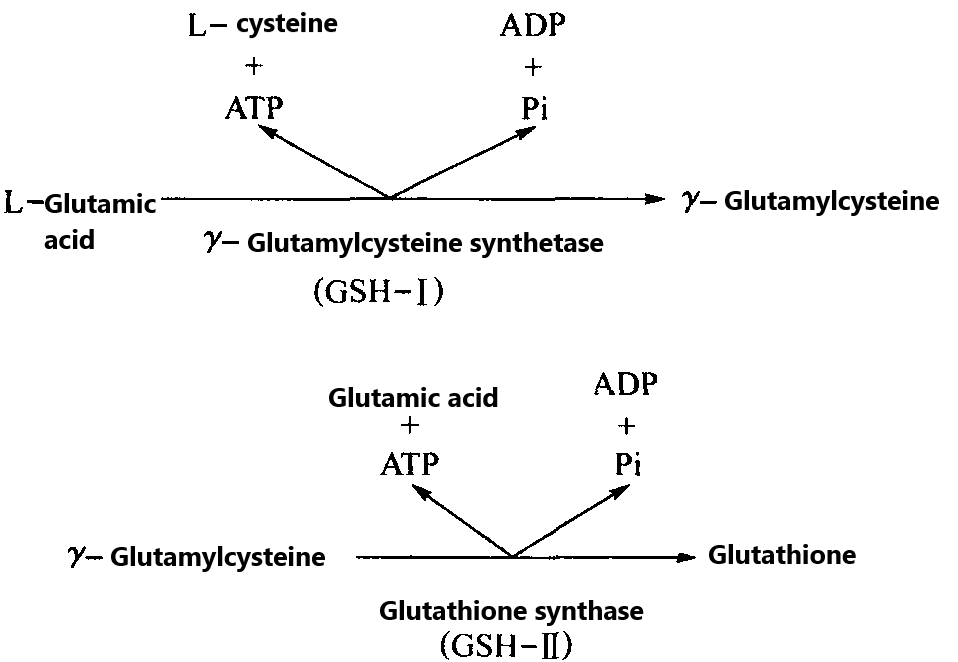
Glutathione biosynthesis, which directly controlled by its synthase system rather than on the ribosome like protein synthesis, consists of the following two reactions, as shown in Figure 2.
As early as 1921, Hopkins first discovered glutathione, and glutathione divided into two categories: reduced (GSH) and oxidized (GSSG).
GSH exists in all biological cells, and the content of yeast, wheat germ and animal liver is extremely high, reaching 100-1000mg /100g.
According to recent data, S.c ChemicalbookerevisiaeJN – 5-8 strains of GSH content of up to 3058 mg / 100 g.
It is present in dry yeast as oxidized glutathione, while human red blood cells are almost entirely reduced glutathione, and GSH can be synthesized in red blood cells.
Glutathione contains an active sulfhydryl – SH, which easily dehydrogenated by oxidation, and two molecules of reduced glutathione (GSH) dehydrogenated into one molecule of oxidized glutathione (GSSG).
In the oxidized type, two tripeptides linked by disulfide bonds. The one that plays an important physiological role in organisms reduced glutathione, and GSSG needs to reduced to GSH to have physiological activity.
Physicochemical properties of glutathione
The molecular weight of glutathione is 307.33, the melting point is 189 ~ 193℃(decomposition), the crystal is colorless transparent slender column, and the isoelectric point is 5.93.
It is soluble in water, dilute alcohol, liquid ammonia and methylformamide, but insoluble in alcohol, ether and acetone. In vivo, only GSH has physiological activity, while GSSG needs to reduced to play its important physiological functions.
GSH not easy to preserve under high water activity, and only when the water activity controlled below 0.3 can it stably preserved for a long time.
Studies have shown that in the aqueous solution of vitamin C containing GSH (pH3.3), due to the strong reduction of vitamin C, the GSH in the solution will not oxidized to GSSG, but its decomposition rate accelerated;
GSSG in vitamin C aqueous solution will not transformed into GSH, and the preservation stability is very good.
In addition, orally ingested GSSG can reduced to GSH in the upper part of the small intestine, and absorbed by γ-GTP(decomposition of GSH into glutamate and Cys-Gly) and dipeptidase on the surface of small intestinal epithelial cells, which can also play its important physiological functions.
Glutathione is widely found in plants and animals, and the content of glutathione in yeast, wheat germ and animal liver is very high, reaching 100-1000mg /100g.
It is also rich in human and animal blood, such as human blood containing 26 ~ 34mg/100g, chicken blood containing 58 ~ 73mg/100g, pig blood containing 10 ~ 15mg/100g, dog blood containing 14 ~ 22mg/100g.
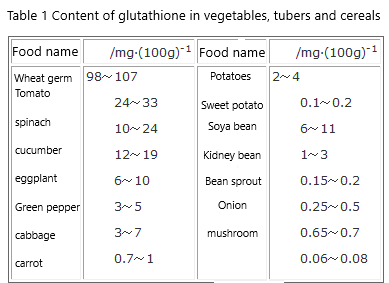
GSH is also found in many vegetables, tubers and cereals (see Table 1).
Food additive
- 1, added to flour products, can play a reducing role.
- 2, add it to yogurt and infant food, equivalent to vitamin C, can play the role of stabilizer.
- 3, Mix it into the fish cake to prevent the color from deepening.
- 4, added to meat products and cheese and other foods, with the effect of strengthening flavor.
Foods rich in glutathione
Onion, garlic, tomato, fish, shrimp, mutton, chili.
Reduced glutathione
Reduced glutathione (GSH) is an important substance in the cell, composed of glutamic acid, cysteine and glycine, containing sulfhydryl group, plays an important role in the maintenance of cellular biological functions, has a variety of biochemical functions, including participation in the tricarboxylic acid cycle and sugar metabolism, is a coenzyme of glyceraldehyde phosphate dehydrogenase and triose phosphate dehydrogenase, can activate a variety of enzymes, Promote the metabolism of sugar, fat and protein, affect the metabolic process of cells;
Through the combination of sulfhydryl groups with free radicals and electrophilic groups in the body, they transformed into acids that can easily metabolized, accelerate the excretion of free radicals, prevent cell damage, reduce the toxic effects of chemotherapy and radiotherapy, and protect the renal tubules from the damage of cisplatin.
GSH can combined with hepatotropic toxicants, protect the synthesis, detoxification and inactivation of hormones in the liver, promote the metabolism of cholic acid, and facilitate the digestive tract to absorb fat and fat-soluble vitamins.
It is suitable for reducing the toxic effects of chemotherapy and radiotherapy, especially high-dose chemotherapy.
Or for the treatment of various hypoxemia, such as acute anemia, acute respiratory distress syndrome, sepsis, etc.;
It can used for the treatment of liver diseases, including liver damage caused by virus, drug, ethanol and other chemical toxicity.
In addition, reduced glutathione can also used for the adjuvant treatment of organophosphorus, amino or nitroaromatic compound poisoning.
It also has therapeutic effect on acute drug-induced kidney injury, uremia, diabetic complications and neuropathy.
Antidote
Glutathione has a broad spectrum of detoxification, can combined with toxic compounds such as acrylonitrile, fluoride, carbon monoxide, heavy metal ions or carcinogens into the body, and promote their excretion, can used for the treatment of the above-mentioned substances poisoning.
Antiallergic agent
It has anti-allergic effect and can treat allergies caused by the imbalance of acetylcholine and cholinesterase in the human body.
Liver-protecting agent
It can protect the liver and inhibit the formation of fatty liver. It can not only used as liver protection agent, but also as feed additive. It can protect the liver of fish and cattle. In the culture, overstocking and unclean feed often lead to liver dysfunction in fish and dairy cattle, adding glutathione can improve liver function.
Glutathione depletion
In glutathione binding, glutathione catalyzed by glutathione-S-transferase to bind foreign chemicals or their metabolites, thereby reducing toxicity and increasing polarity, which is an important detoxification pathway in biotransformation.
When the dose of the foreign chemical suitable for this reaction large, glutathione may depleted and metabolic saturation occurs, so that the amount of binding per unit time no longer increases with the dose of the foreign chemical.
As a result, the toxicity of the corresponding foreign chemical greatly increased, and its dose-response curve very different from that at low doses, and its toxicological kinetics also shows nonlinear dynamics.
Glutathione depletion occurs when a dose of a foreign chemical is too high, or when another foreign chemical competes for the binding, or when glutathione supply reduced due to poor nutrition or tissue damage.
Depletion of glutathione in either case can render a tolerated dose toxic.
antioxidant
Many biochemical reactions in human body enzymatic catalyzed reactions. Most of these enzymes use sulfhydryl group as the active group, and the status of sulfhydryl group determines the activation and inhibition of enzyme activity.
Glutathione is the natural activator of these enzymes in cells, and contains sulfhydryl groups that can reduce H2O2 produced by human cell metabolism to H2O and remove free radicals in the human body.
Free radicals can damage cell membranes, promote aging, and cause tumors or arteriosclerosis. It has an anti-peroxidation effect on human cells, and can also improve the antioxidant capacity of the skin and make the skin shine.
Human aging, infection, poisoning, exogenous toxins, oxidative stress, and electrophilic compound attack can all reduce the level of GSH in the cytoplasm. This phenomenon occurs in the very early stage of apoptosis, and the degradation process can observed in the early stage of apoptosis. Therefore, the detection of glutathione level can early judge and detect apoptosis.
Glutathione has the function of eliminating the generation of oxidized lipids, and has the effect of anti-lipid oxidation, and can also prevent the decomposition of flavor nucleotides (inosine acid, guanylate) in food (fish cake, sausage, soy sauce, etc.) and lose fresh and thick taste.
Preparation of glutathione
Since the first patent for the preparation of reduced glutathione (GSH) from yeast published in 1938, a large number of patent applications have filed since then.
In general, there are four methods for the preparation of GSH: solvent extraction, enzyme, fermentation and chemical synthesis.
At present, it mainly extracted from the cultivation of high content GSH yeast, and many biochemical companies such as domestic manufacturer Gutuo Biology and many universities and research units are trial-producing and developing.
The extraction method and enzymatic method mostly made of wheat germ as raw material, by adding appropriate solvent or combining with amylase and protease treatment, and then by centrifugation, separation and refining. Its process is simple, see Figure 4 below.
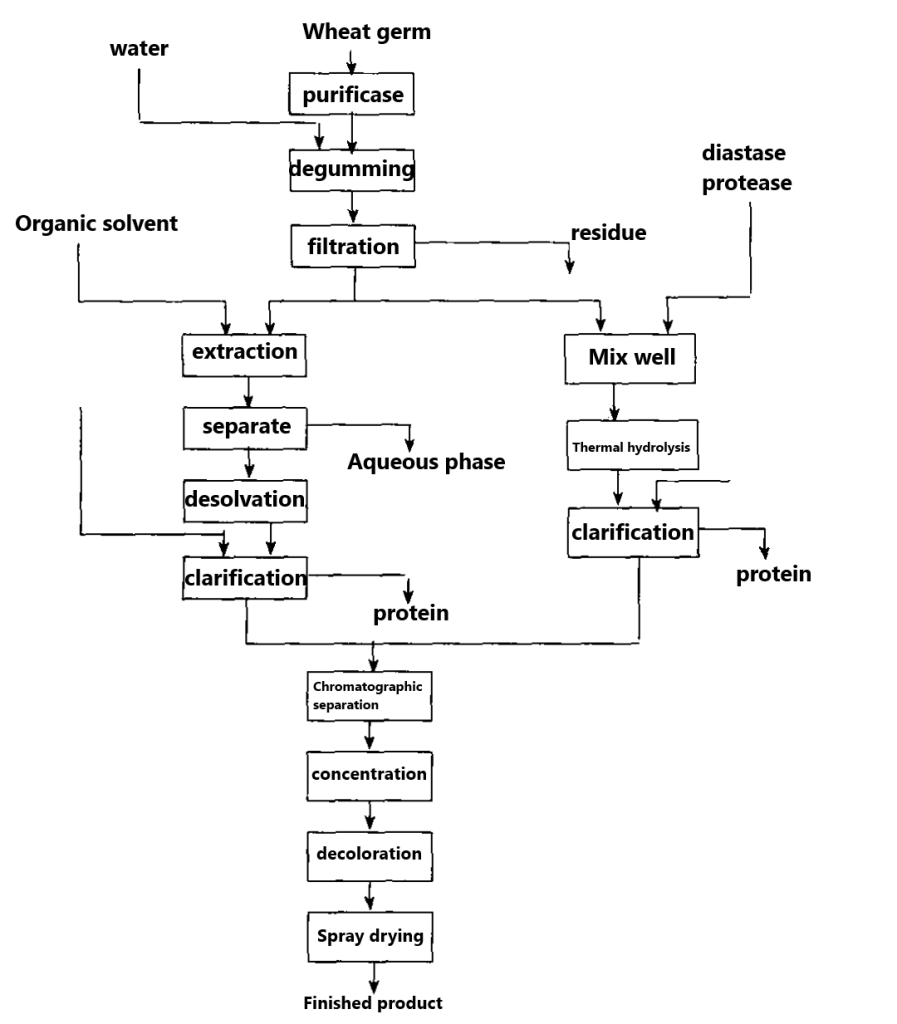
There are two methods to prepare glutathione by biotechnology. One is to breed high-yield yeast strains rich in GSH, and then to isolate and extract it. Another approach is to culture GSH-rich green algae and extract them in a similar way to yeast.
Glutathione for use
Reduced glutathione is a small molecule peptide, a large number of organisms, especially in liver cells, to protect the liver cell membrane, promote the activity of liver enzymes, and with many toxic chemicals combined to play a detoxification role. For drug poisoning, alcohol poisoning and other causes of liver injury, cirrhosis and other diseases have a very good effect.
It has the functions of anti-oxidation, scavenging free radicals, detoxification, enhancing immunity, delaying aging, anti-cancer, anti-radiation and so on.
biochemical reagents, antidotes, mainly used for poisoning heavy metals, acrylonitrile, fluoride, carbon monoxide and organic solvents
Biochemical research
Endogenous antioxidant that plays an important role in the reduction of reactive oxygen species formed during cell metabolism and sudden respiration.
Glutathione-s-transferase catalyzes the formation of glutathione sulfide with heteroorganisms, leukotrienes, and other molecules with electrophilic centers.
Glutathione also forms disulfide bonds with cysteine residues in proteins. Through these mechanisms, it can have the singular effect of reducing the effectiveness of anticancer agents.